Structure
| InChI Key | SDEAXTCZPQIFQM-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C26H24N8O2 |
| Molecular Weight | 480.53 |
| AlogP | 5.09 |
| Hydrogen Bond Acceptor | 10.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 110.85 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 5.0 |
| Heavy Atoms | 36.0 |
Pharmacology
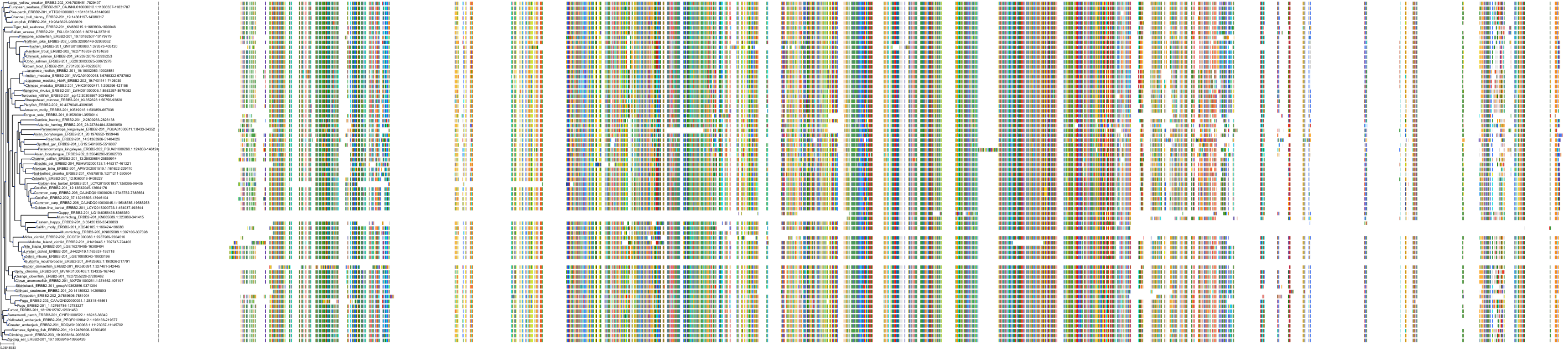
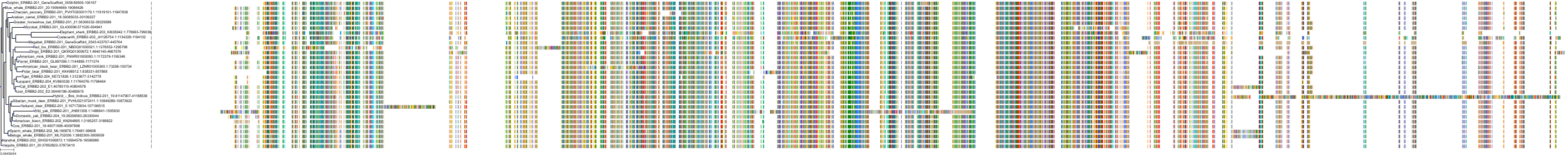
Target Conservation
|
Protein: Receptor protein-tyrosine kinase erbB-2 Description: Receptor tyrosine-protein kinase erbB-2 Organism : Homo sapiens P04626 ENSG00000141736 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3989868 |
| DrugBank | DB11652 |
| DrugCentral | 5389 |
| FDA SRS | 234248D0HH |
| Guide to Pharmacology | 9922 |
| PubChem | 51039094 |
| SureChEMBL | SCHEMBL1193050 |
| ZINC | ZINC000068250462 |