| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L01XK04 |
| UNII | 9QHX048FRV |
Structure
| InChI Key | HWGQMRYQVZSGDQ-HZPDHXFCSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C19H14F2N6O |
| Molecular Weight | 380.36 |
| AlogP | 2.63 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 2.0 |
| Polar Surface Area | 88.49 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 28.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Poly [ADP-ribose] polymerase 2 inhibitor | INHIBITOR | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Transferase
|
2.5-3.2 | 0.57-7.2 | - | 0.85-1.2 | 91 |
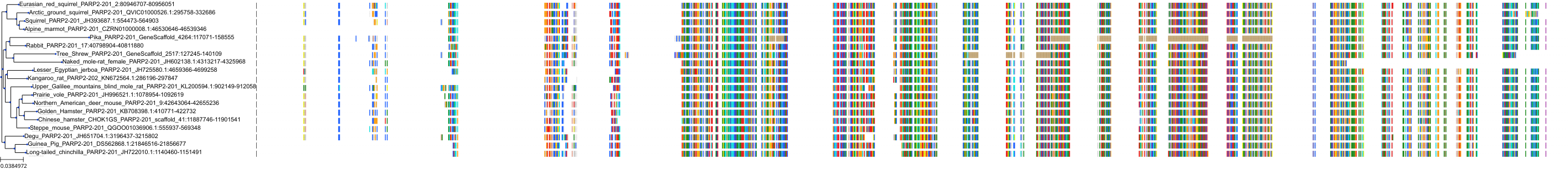
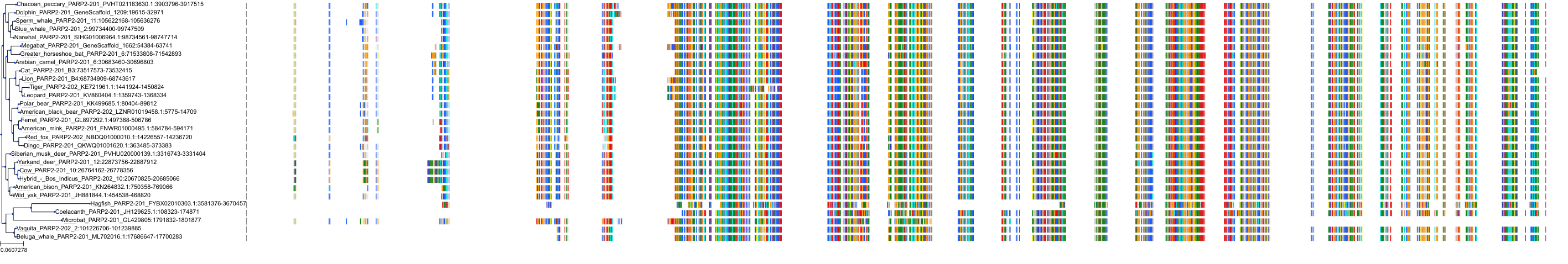
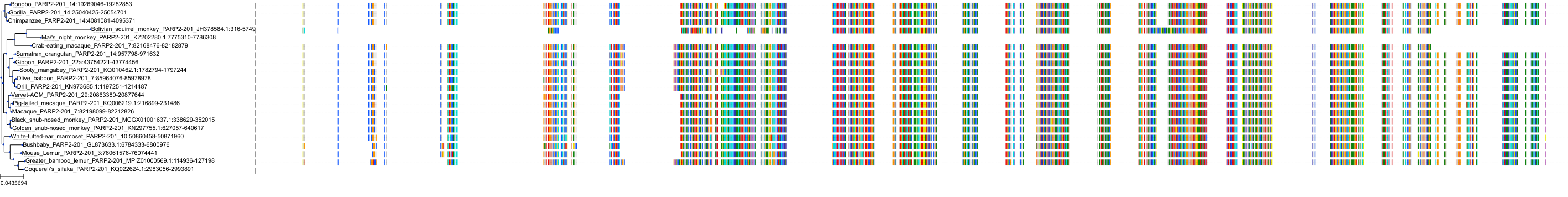
Target Conservation
|
Protein: Poly [ADP-ribose] polymerase-1 Description: Poly [ADP-ribose] polymerase 1 Organism : Homo sapiens P09874 ENSG00000143799 |
||||
|
Protein: Poly [ADP-ribose] polymerase 2 Description: Poly [ADP-ribose] polymerase 2 Organism : Homo sapiens Q9UGN5 ENSG00000129484 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3137320 |
| DrugBank | DB11760 |
| DrugCentral | 5300 |
| FDA SRS | 9QHX048FRV |
| Guide to Pharmacology | 8313 |
| PDB | 2YQ |
| PubChem | 135565082 |
| SureChEMBL | SCHEMBL2299348 |
| ZINC | ZINC000072318110 |
 Cricetulus griseus
Cricetulus griseus
 Homo sapiens
Homo sapiens