Structure
| InChI Key | IWXUVYOOUMLUTQ-CZUORRHYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C20H25N7O2 |
| Molecular Weight | 395.47 |
| AlogP | 1.5 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 4.0 |
| Polar Surface Area | 101.82 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 29.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Phosphodiesterase
Phosphodiesterase 9
Phosphodiesterase 9A
|
- | 8.3-49 | - | - | - |
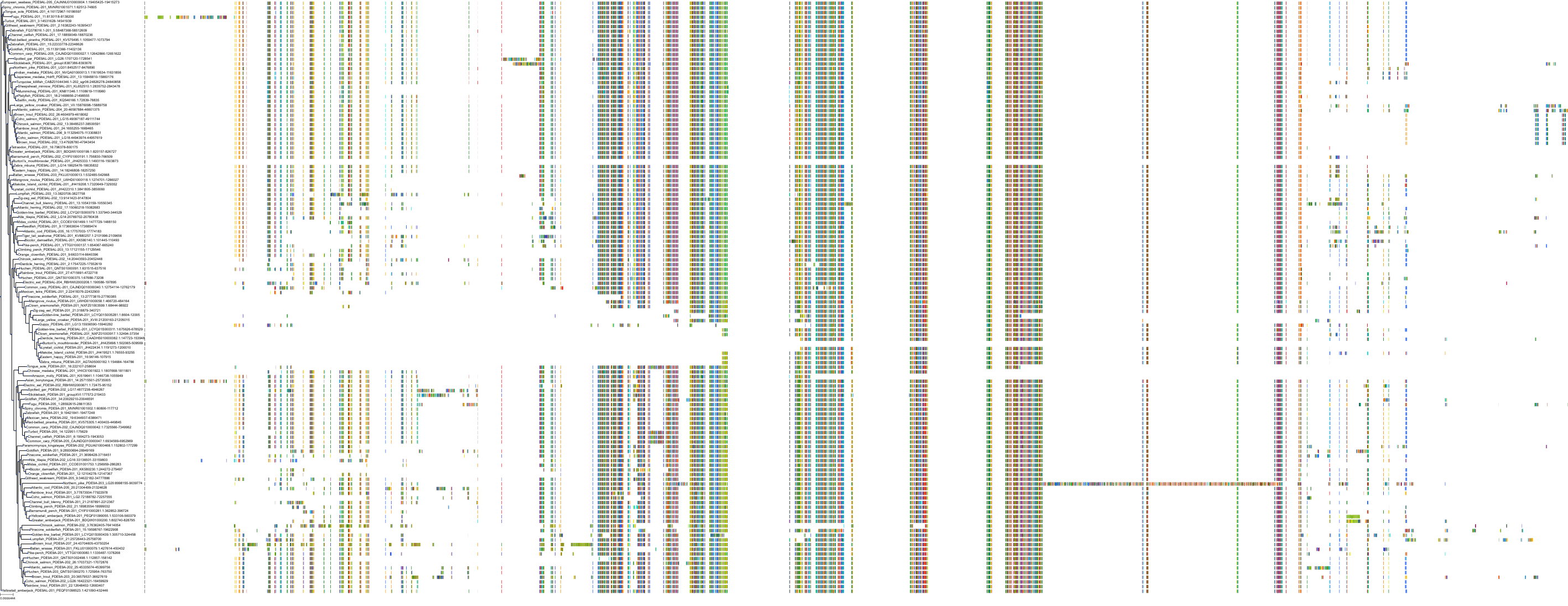
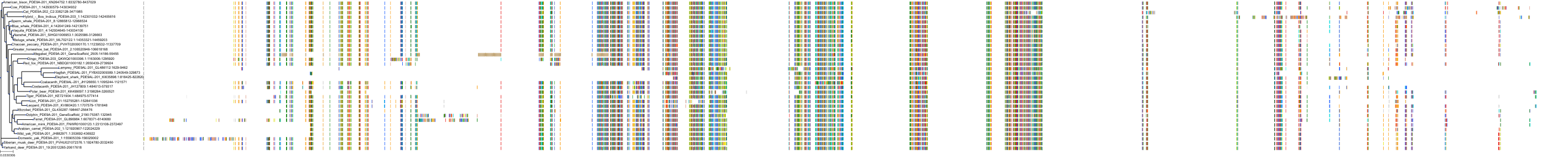
Target Conservation
|
Protein: Phosphodiesterase 9A Description: High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A Organism : Homo sapiens O76083 ENSG00000160191 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL2179105 |
| DrugBank | DB11953 |
| FDA SRS | 7N969W8Y4O |
| PDB | 7RG |
| PubChem | 135564558 |
| SureChEMBL | SCHEMBL1716847 |
| ZINC | ZINC000068199983 |
 Homo sapiens
Homo sapiens