Structure
| InChI Key | ZUHZNKJIJDAJFD-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C20H28N8O4S |
| Molecular Weight | 476.56 |
| AlogP | 1.46 |
| Hydrogen Bond Acceptor | 11.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 10.0 |
| Polar Surface Area | 144.23 |
| Molecular species | ACID |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 33.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Phosphodiesterase
Phosphodiesterase 5
Phosphodiesterase 5A
|
- | 0.5012 | - | - | - |
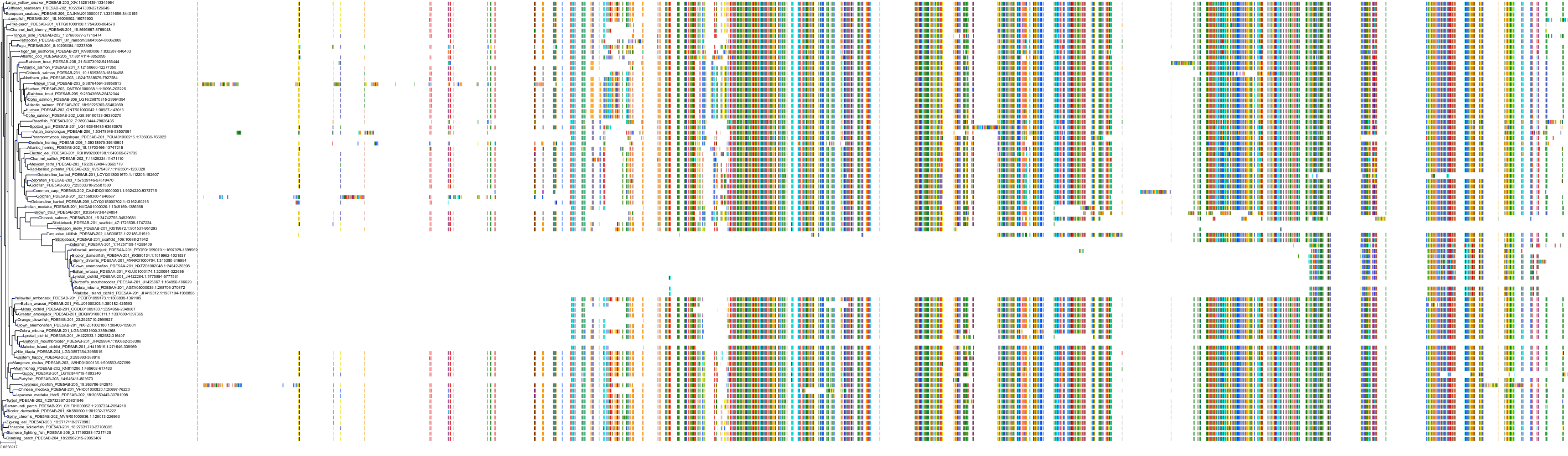
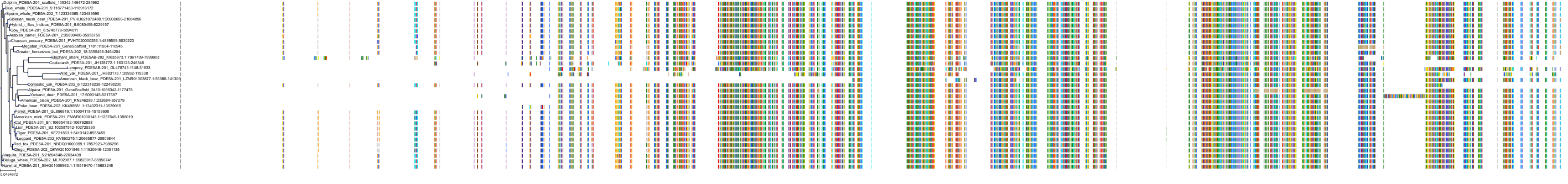
Target Conservation
|
Protein: Phosphodiesterase 5A Description: cGMP-specific 3',5'-cyclic phosphodiesterase Organism : Homo sapiens O76074 ENSG00000138735 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL4210847 |
| DrugBank | DB11736 |
| FDA SRS | 2S27T3DSZ3 |
| Guide to Pharmacology | 8377 |
| PubChem | 11465695 |
| SureChEMBL | SCHEMBL331279 |
 Homo sapiens
Homo sapiens