| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | M09AX11 |
| UNII | 28K6I5M16G |
Structure
| InChI Key | YTFHCXIPDIHOIA-DHZHZOJOSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C27H30N2O2 |
| Molecular Weight | 414.55 |
| AlogP | 6.15 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 55.12 |
| Molecular species | ACID |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 31.0 |
Pharmacology
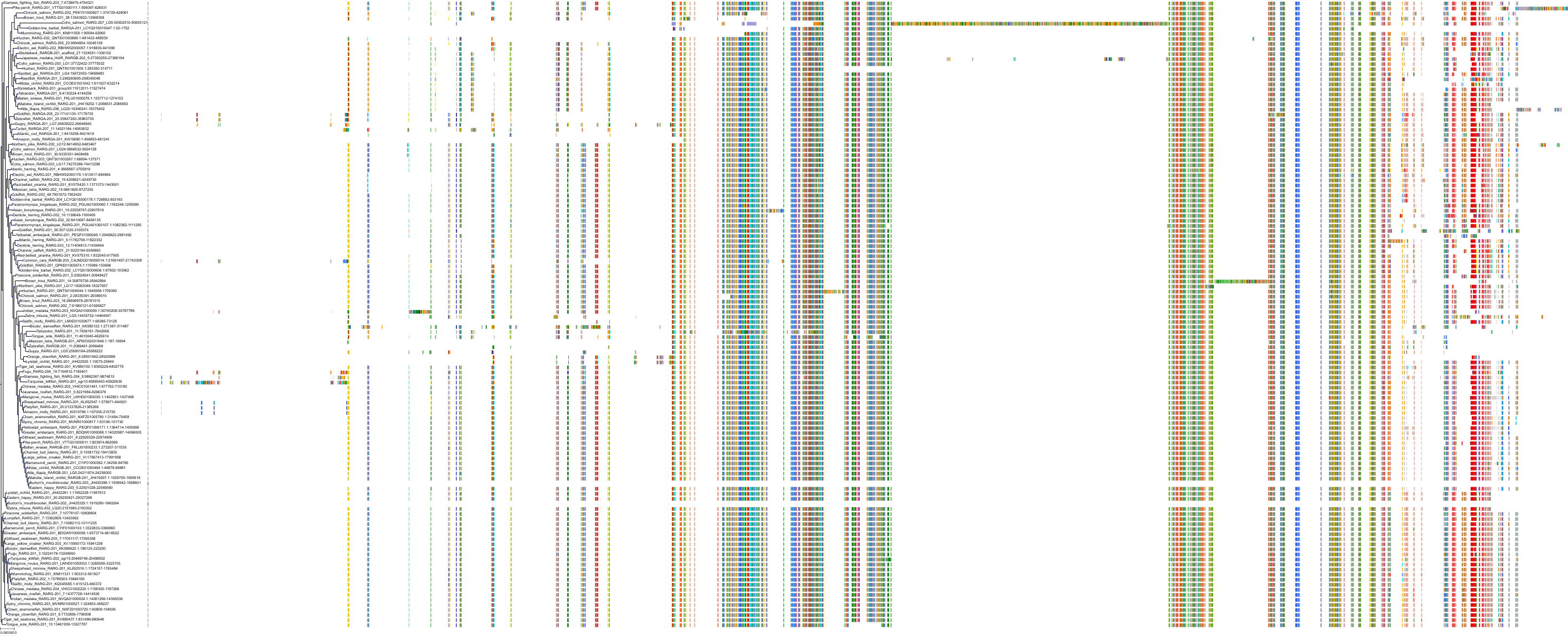
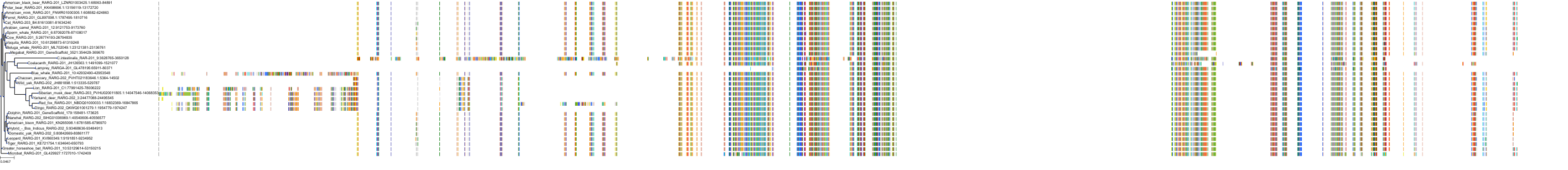
Target Conservation
|
Protein: Retinoic acid receptor gamma Description: Retinoic acid receptor gamma Organism : Homo sapiens P13631 ENSG00000172819 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL2105648 |
| DrugBank | DB12320 |
| FDA SRS | 28K6I5M16G |
| Guide to Pharmacology | 8276 |
| PubChem | 10295295 |
| SureChEMBL | SCHEMBL4658931 |
| ZINC | ZINC000038467831 |