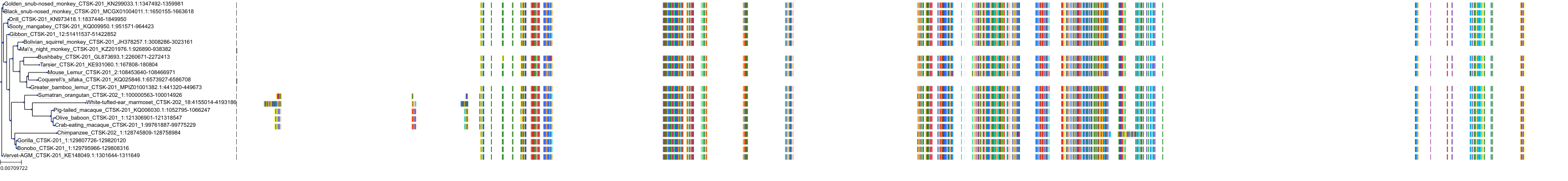| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | N673F6W2VH |
| EPA CompTox | DTXSID40209075 |
Structure
| InChI Key | FWIVDMJALNEADT-SFTDATJTSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C25H27F4N3O3S |
| Molecular Weight | 525.57 |
| AlogP | 4.63 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 9.0 |
| Polar Surface Area | 99.06 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 36.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Cathepsin K inhibitor | INHIBITOR | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Protease
Cysteine protease
Cysteine protease CA clan
Cysteine protease C1A family
|
- | 0.2-795 | - | 0.18-125.89 | 19 |
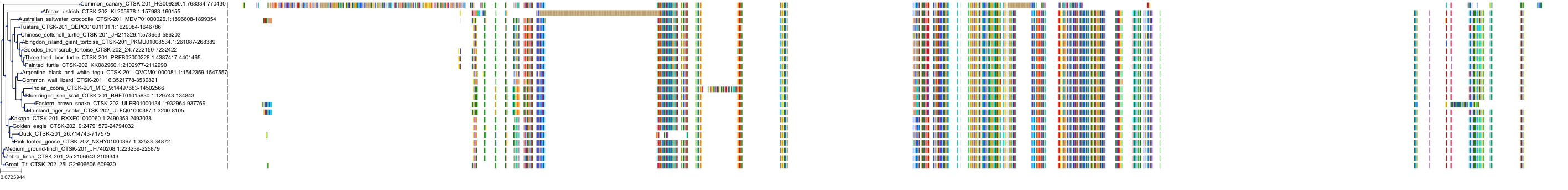
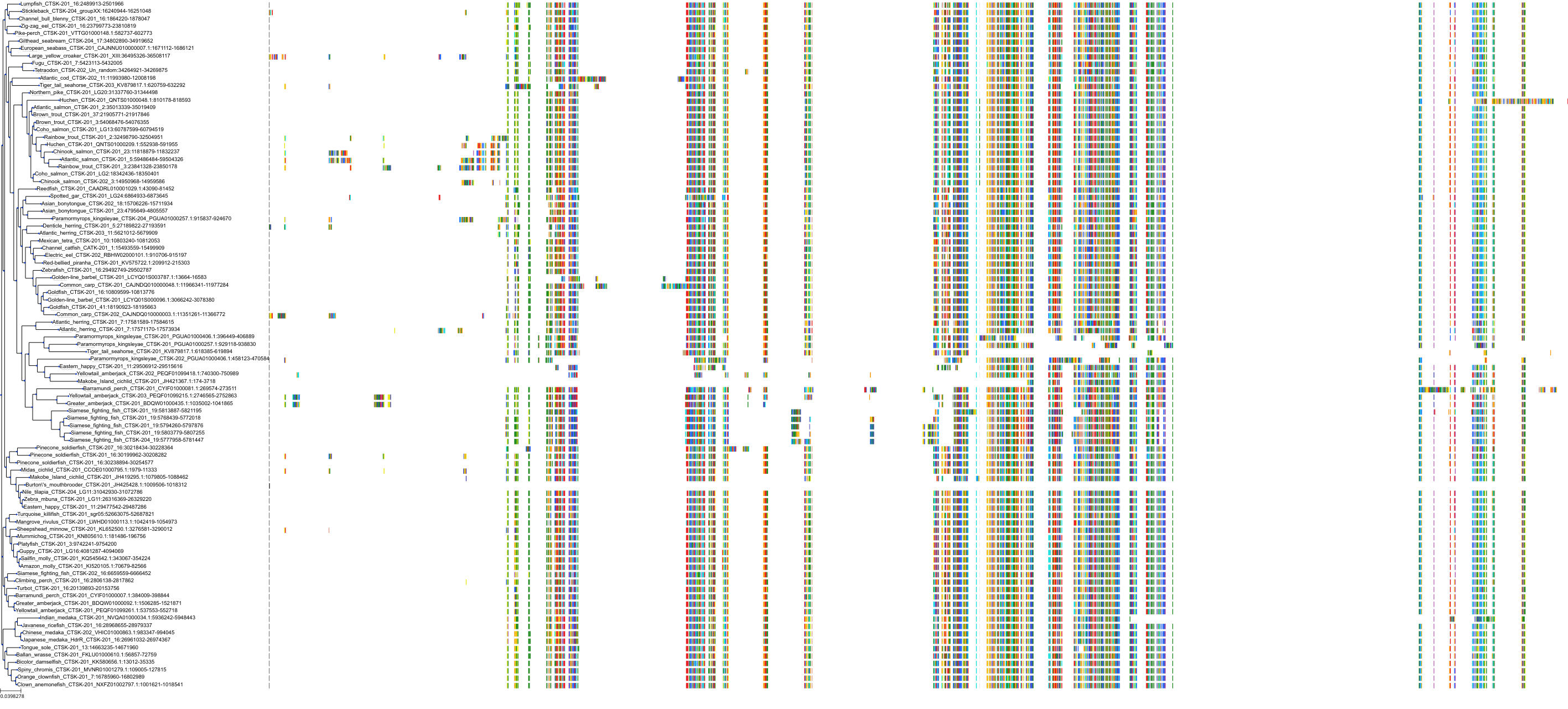
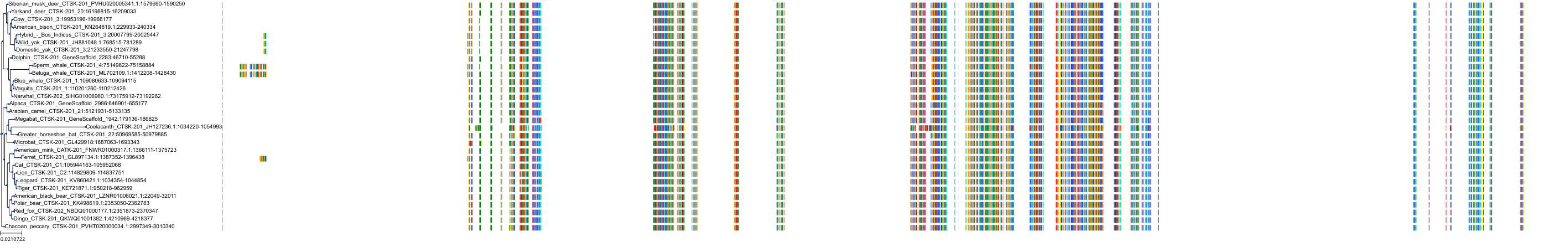
Target Conservation
|
Protein: Cathepsin K Description: Cathepsin K Organism : Homo sapiens P43235 ENSG00000143387 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL481611 |
| DrugBank | DB06670 |
| FDA SRS | N673F6W2VH |
| Guide to Pharmacology | 6478 |
| PubChem | 10152654 |
| SureChEMBL | SCHEMBL1496266 |
| ZINC | ZINC000042893657 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Oryctolagus cuniculus
Oryctolagus cuniculus
 Trypanosoma cruzi
Trypanosoma cruzi