| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | R9PHW59SFN |
| EPA CompTox | DTXSID5045176 |
Structure
| InChI Key | HRRBJVNMSRJFHQ-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C24H28N2O3 |
| Molecular Weight | 392.5 |
| AlogP | 3.41 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 45.17 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 29.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Alpha-1d adrenergic receptor antagonist | ANTAGONIST | PubMed PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Membrane receptor
Family A G protein-coupled receptor
Small molecule receptor (family A GPCR)
Monoamine receptor
Adrenergic receptor
|
- | 55.2-634 | 11.75-177.83 | 1.202-39 | - |
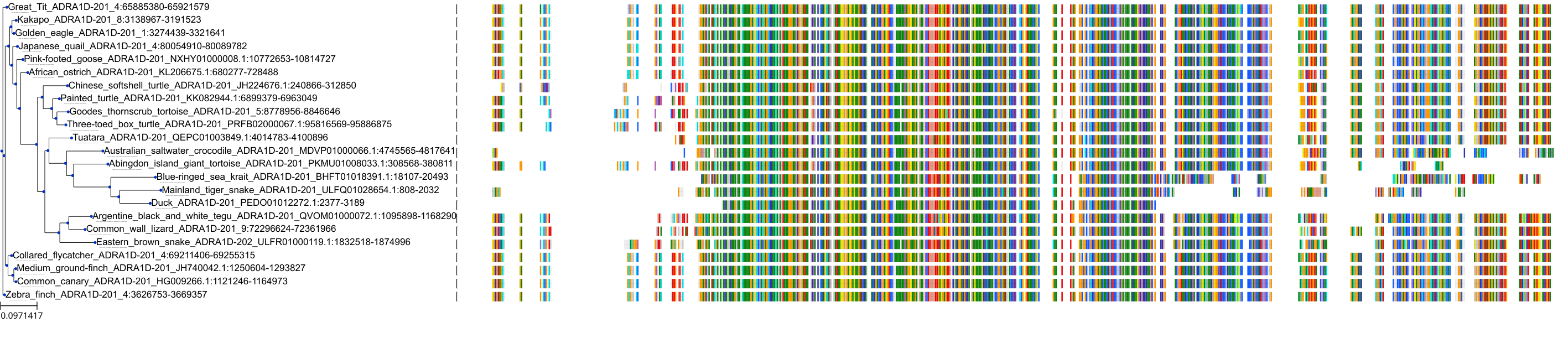
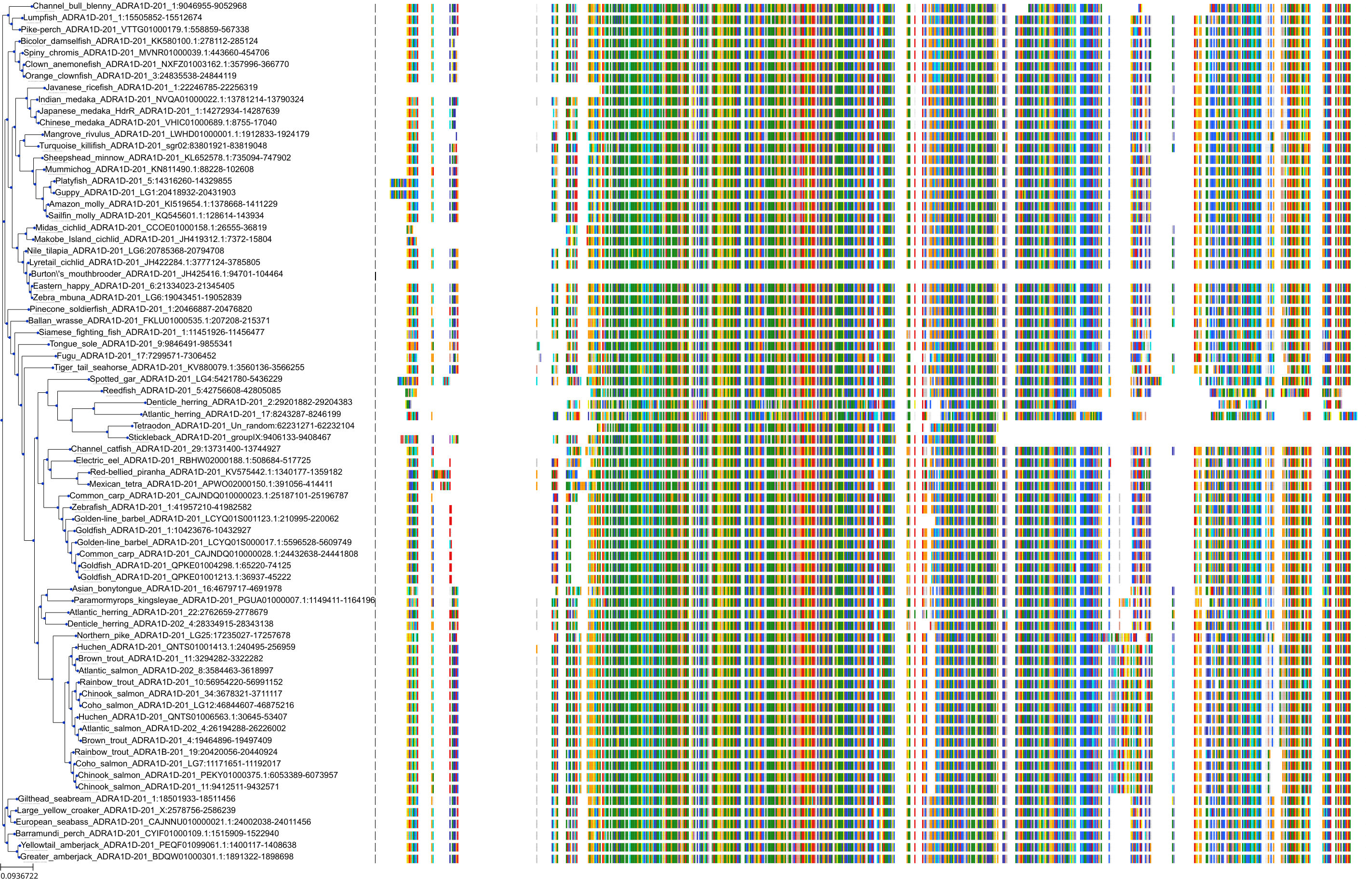
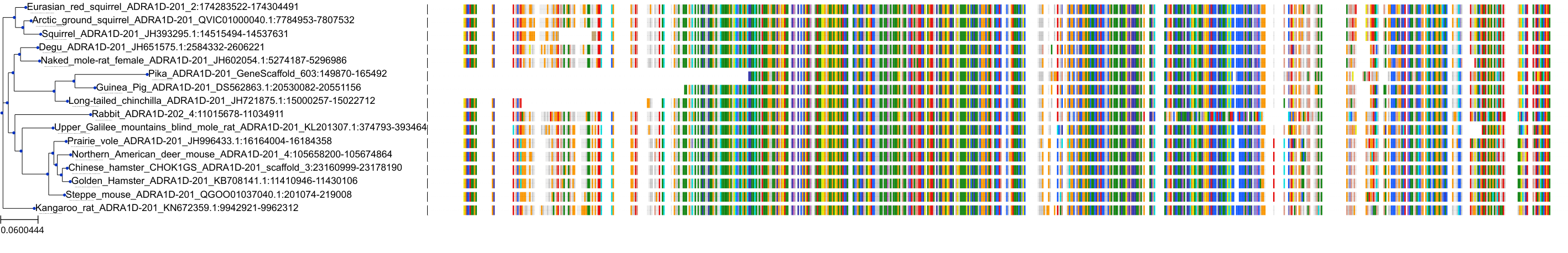
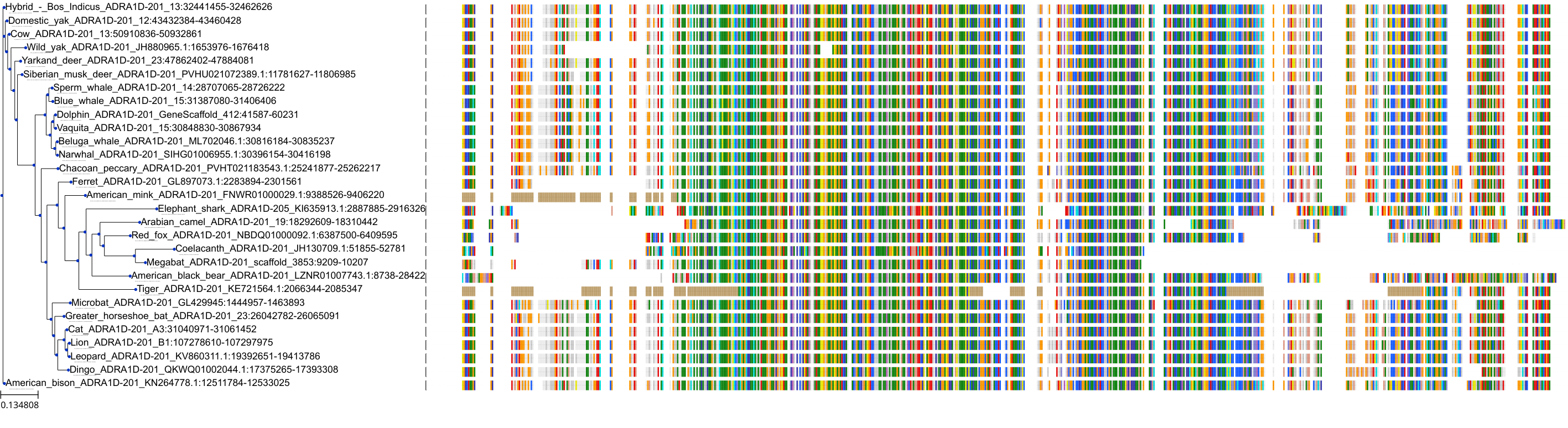
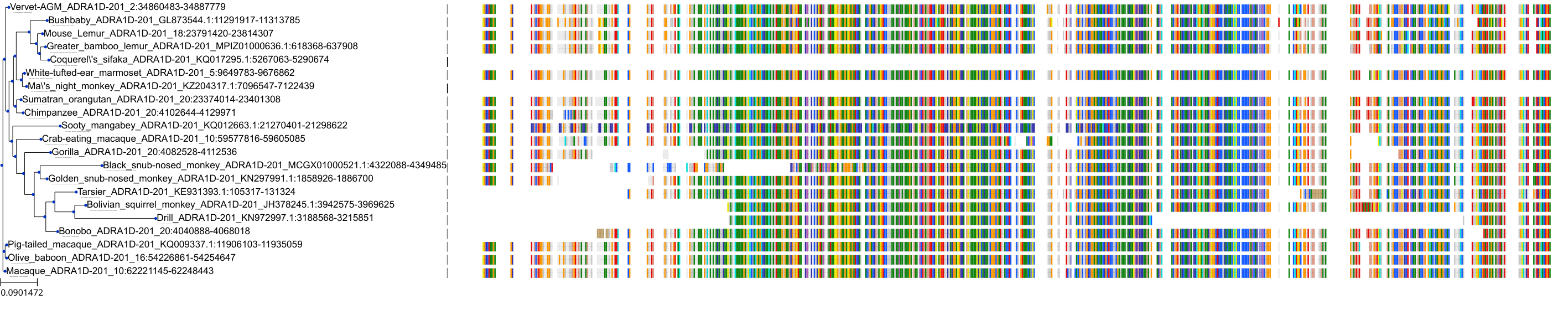
Target Conservation
|
Protein: Alpha-1d adrenergic receptor Description: Alpha-1D adrenergic receptor Organism : Homo sapiens P25100 ENSG00000171873 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 31891 |
| ChEMBL | CHEMBL142635 |
| DrugBank | DB12092 |
| DrugCentral | 1873 |
| FDA SRS | R9PHW59SFN |
| PubChem | 4418 |
| SureChEMBL | SCHEMBL113215 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus