| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | C01CX08 |
| UNII | C6T4514L4E |
| EPA CompTox | DTXSID9046445 |
Structure
| InChI Key | WHXMKTBCFHIYNQ-SECBINFHSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C14H12N6O |
| Molecular Weight | 280.29 |
| AlogP | 1.36 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 3.0 |
| Polar Surface Area | 113.43 |
| Molecular species | ACID |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 21.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Phosphodiesterase
Phosphodiesterase 3
Phosphodiesterase 3A
|
- | 8 | - | - | - | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 3
Phosphodiesterase 3B
|
- | 8 | - | - | - |
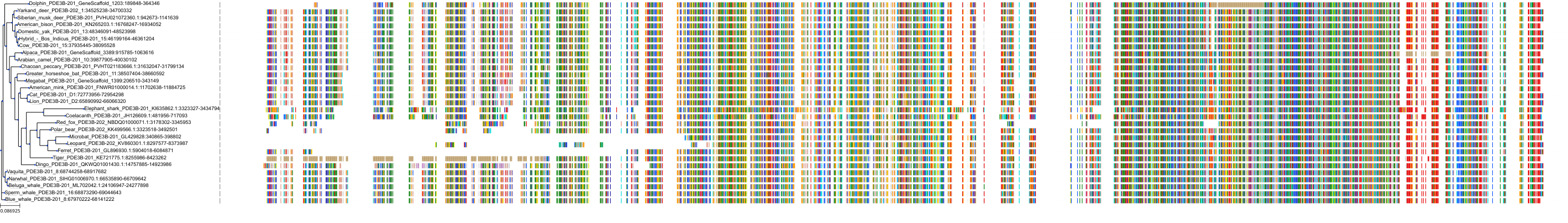
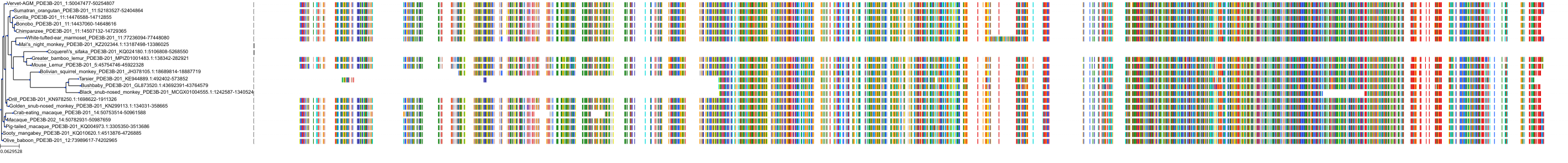
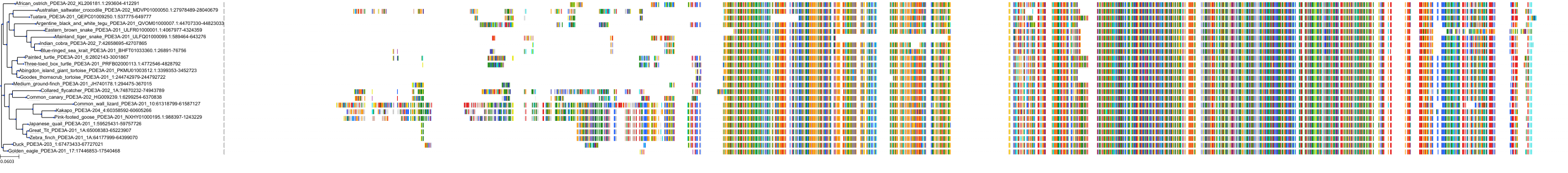
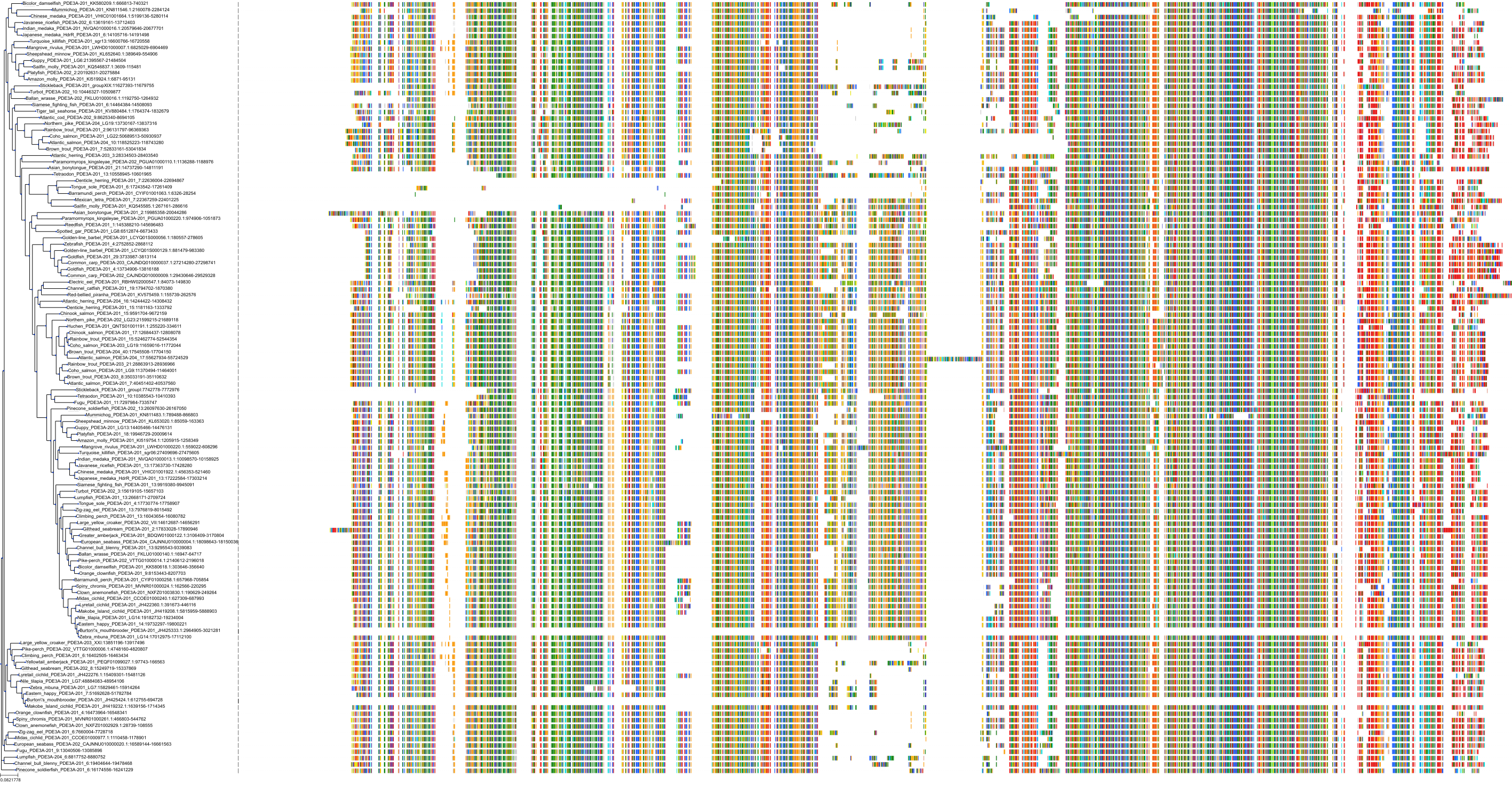
Target Conservation
|
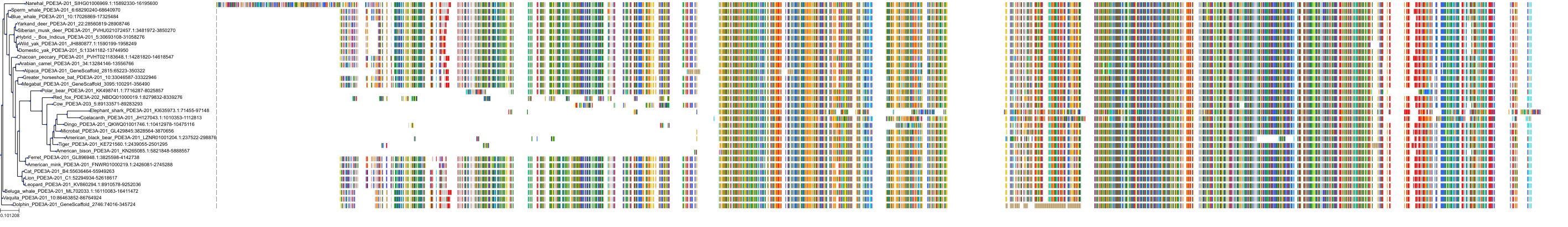
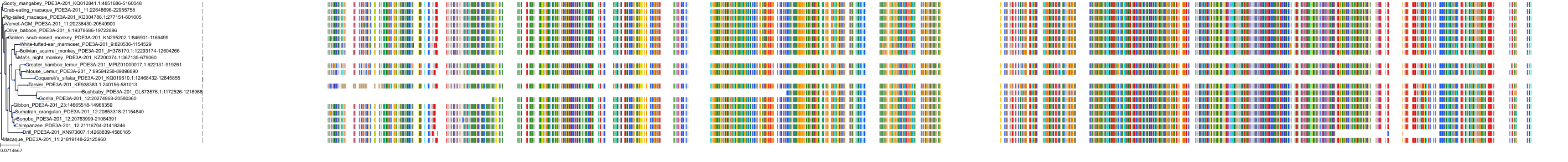
Protein: Phosphodiesterase 3 Description: cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B Organism : Homo sapiens Q13370 ENSG00000152270 |
||||
|
Protein: Phosphodiesterase 3 Description: cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A Organism : Homo sapiens Q14432 ENSG00000172572 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 50567 |
| ChEMBL | CHEMBL2051955 |
| DrugBank | DB00922 |
| DrugCentral | 1576 |
| FDA SRS | C6T4514L4E |
| Human Metabolome Database | HMDB0015058 |
| PharmGKB | PA164749138 |
| PubChem | 3033825 |
| SureChEMBL | SCHEMBL83243 |
| ZINC | ZINC000003915645 |