| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | C01CE03 |
| UNII | C7Z4ITI7L7 |
| EPA CompTox | DTXSID8045147 |
Structure
| InChI Key | ZJKNESGOIKRXQY-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C12H12N2O2S |
| Molecular Weight | 248.31 |
| AlogP | 1.96 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 3.0 |
| Polar Surface Area | 65.72 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 17.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Phosphodiesterase 3 inhibitor | INHIBITOR | BNF |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4A
|
- | - | - | - | 34 | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4B
|
- | - | - | - | 34 | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4C
|
- | - | - | - | 34 | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4D
|
- | - | - | - | 34 |
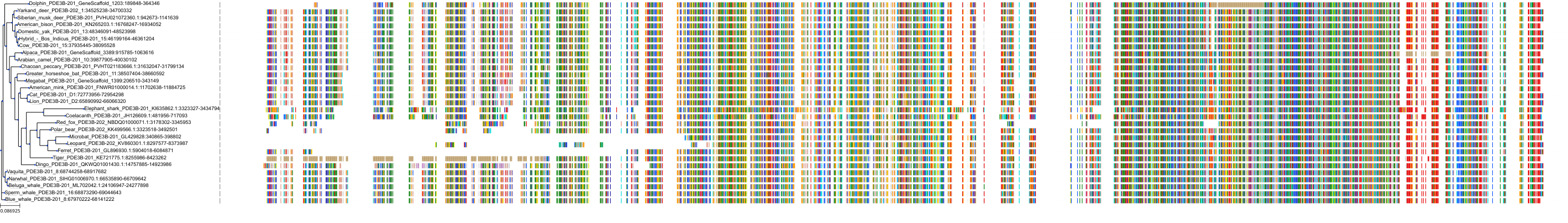
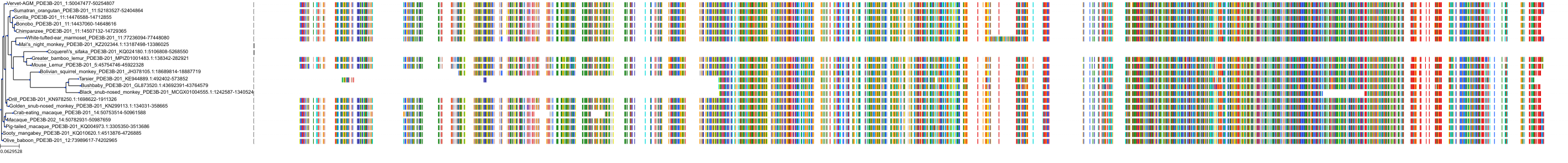
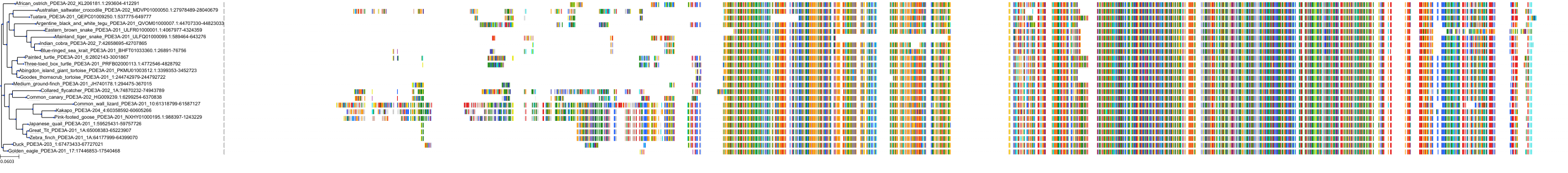
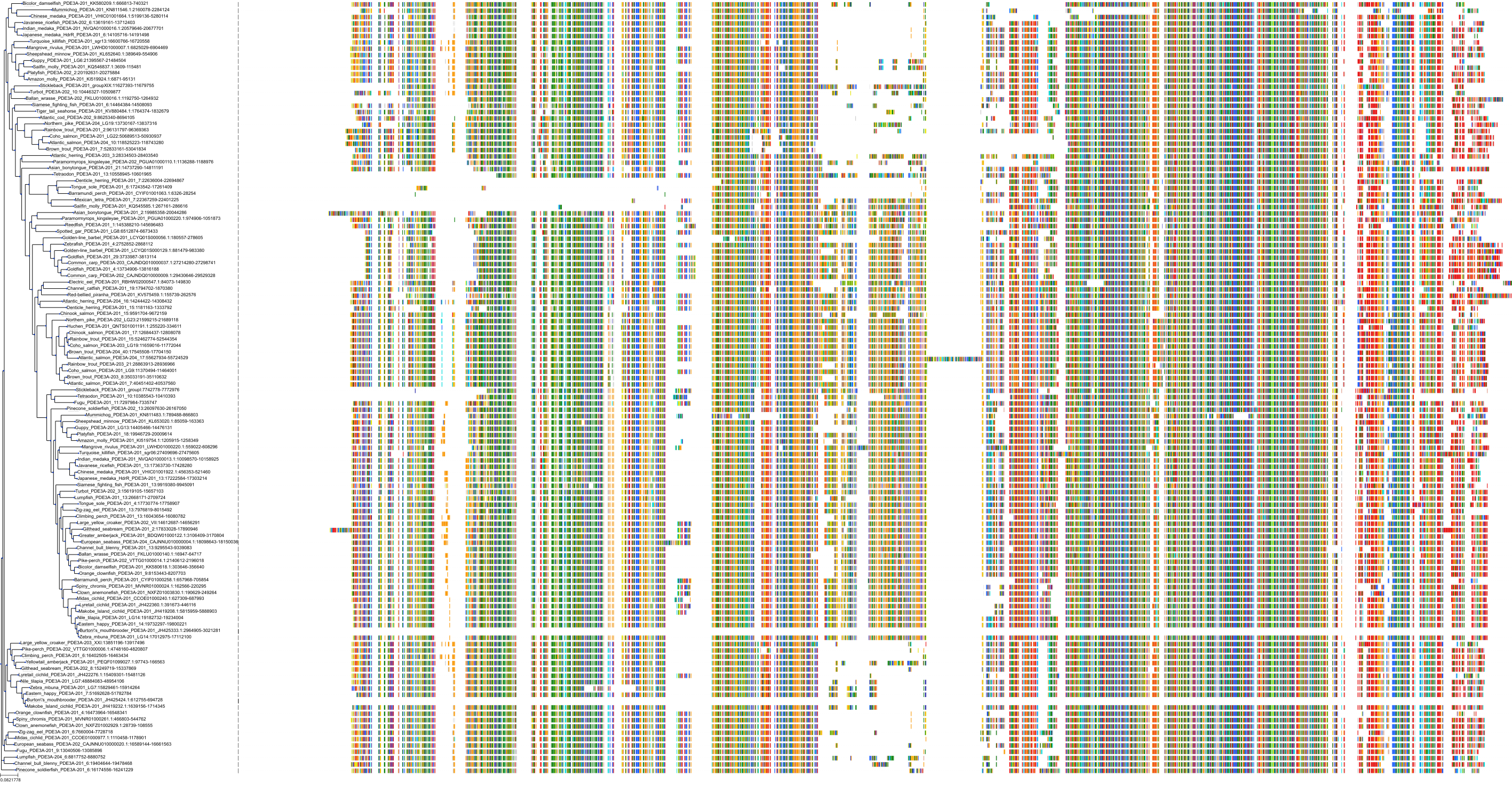
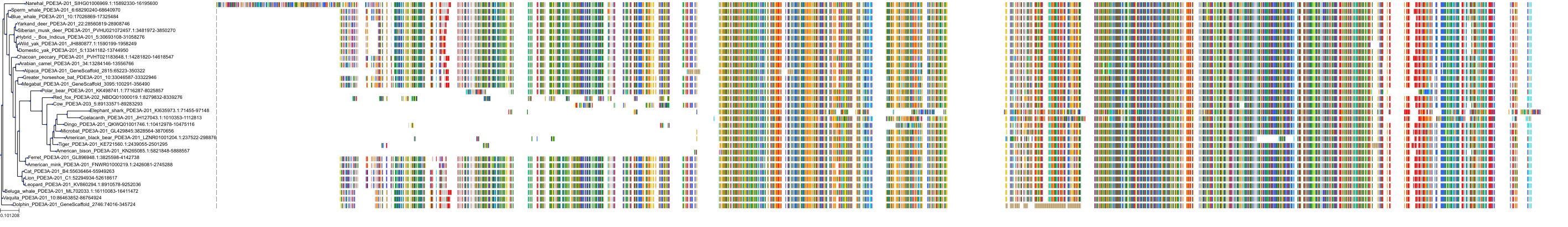
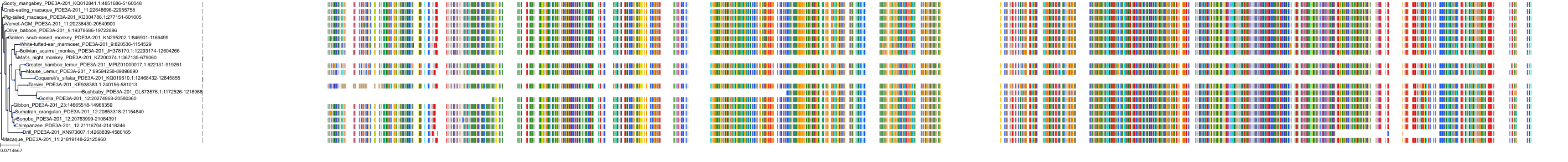
Target Conservation
|
Protein: Phosphodiesterase 3 Description: cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B Organism : Homo sapiens Q13370 ENSG00000152270 |
||||
|
Protein: Phosphodiesterase 3 Description: cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A Organism : Homo sapiens Q14432 ENSG00000172572 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 135010 |
| ChEMBL | CHEMBL249856 |
| DrugBank | DB04880 |
| DrugCentral | 1014 |
| FDA SRS | C7Z4ITI7L7 |
| Human Metabolome Database | HMDB0015599 |
| Guide to Pharmacology | 9063 |
| PharmGKB | PA164768794 |
| PubChem | 53708 |
| SureChEMBL | SCHEMBL44049 |
| ZINC | ZINC000009225358 |
 Canis lupus familiaris
Canis lupus familiaris