| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | A03AD02 |
| UNII | 98QS4N58TW |
| EPA CompTox | DTXSID60161227 |
Structure
| InChI Key | OMFNSKIUKYOYRG-MOSHPQCFSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C24H31NO4 |
| Molecular Weight | 397.52 |
| AlogP | 4.93 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 9.0 |
| Polar Surface Area | 48.95 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 29.0 |
Pharmacology
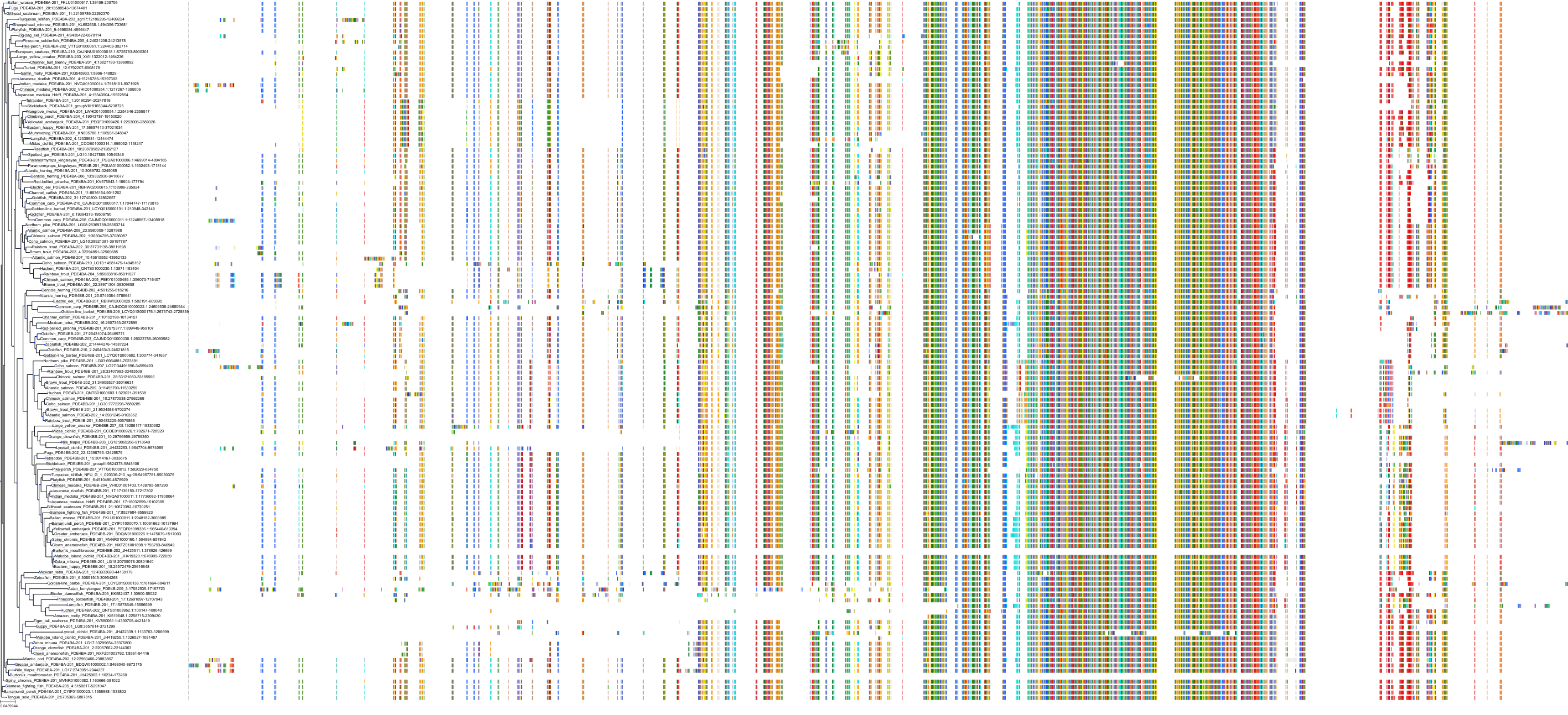
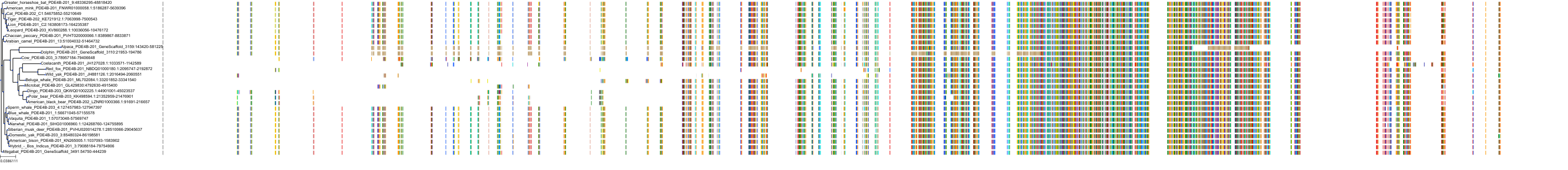
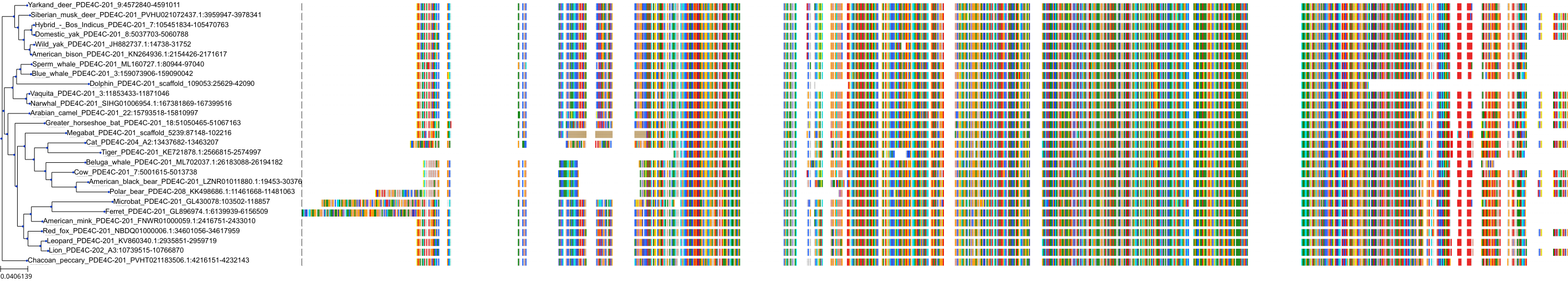
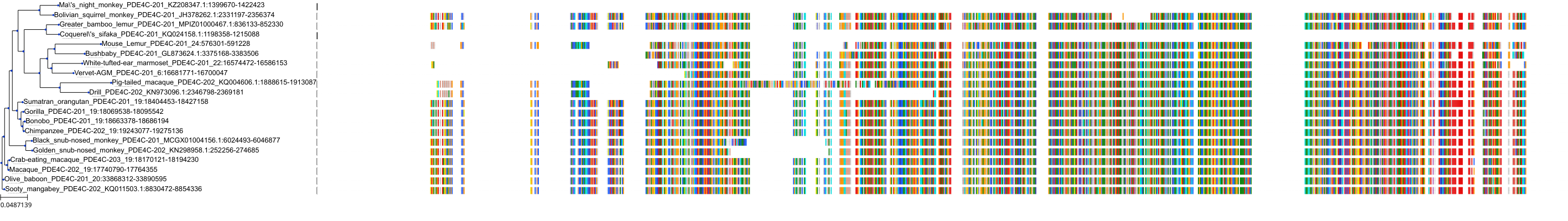
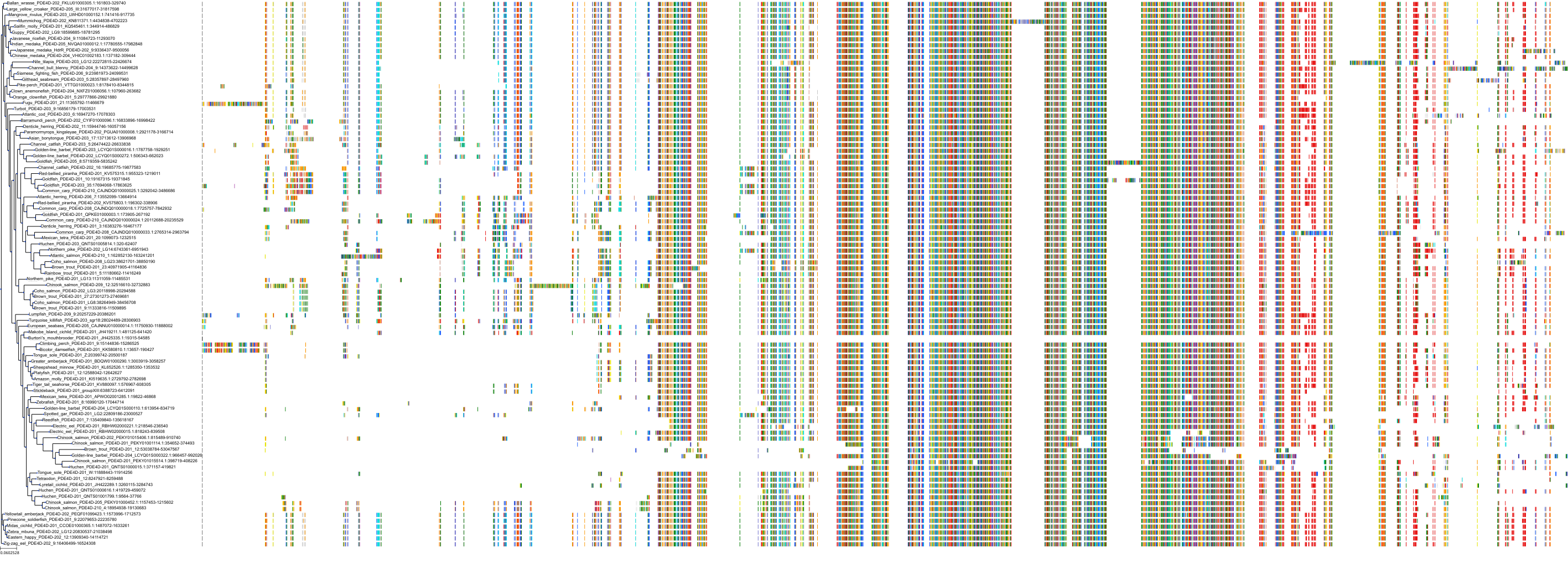
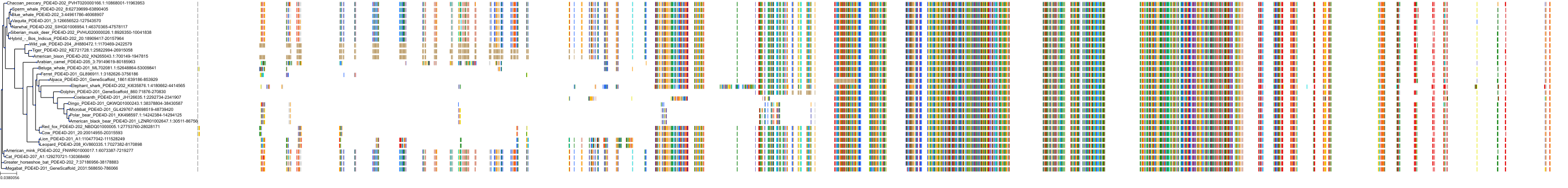
Target Conservation
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4A Organism : Homo sapiens P27815 ENSG00000065989 |
||||
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4B Organism : Homo sapiens Q07343 ENSG00000184588 |
||||
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4C Organism : Homo sapiens Q08493 ENSG00000105650 |
||||
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4D Organism : Homo sapiens Q08499 ENSG00000113448 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 135630 |
| ChEMBL | CHEMBL551978 |
| DrugBank | DB06751 |
| DrugCentral | 969 |
| FDA SRS | 98QS4N58TW |
| Human Metabolome Database | HMDB0015669 |
| PharmGKB | PA165958398 |
| PubChem | 1712095 |
| SureChEMBL | SCHEMBL196438 |
| ZINC | ZINC000100011979 |