| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | 69DY40RH9B |
| EPA CompTox | DTXSID50152604 |
Structure
| InChI Key | QNQZBKQEIFTHFZ-GOSISDBHSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C21H30Cl2N2O5 |
| Molecular Weight | 461.39 |
| AlogP | 4.01 |
| Hydrogen Bond Acceptor | 4.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 14.0 |
| Polar Surface Area | 95.94 |
| Molecular species | ACID |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 30.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Cholecystokinin A receptor antagonist | ANTAGONIST | PubMed |
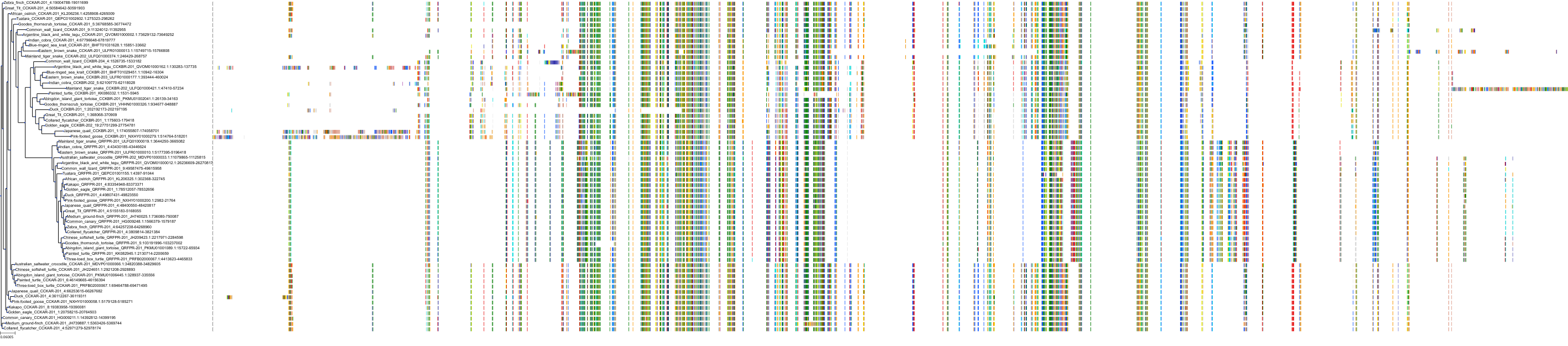
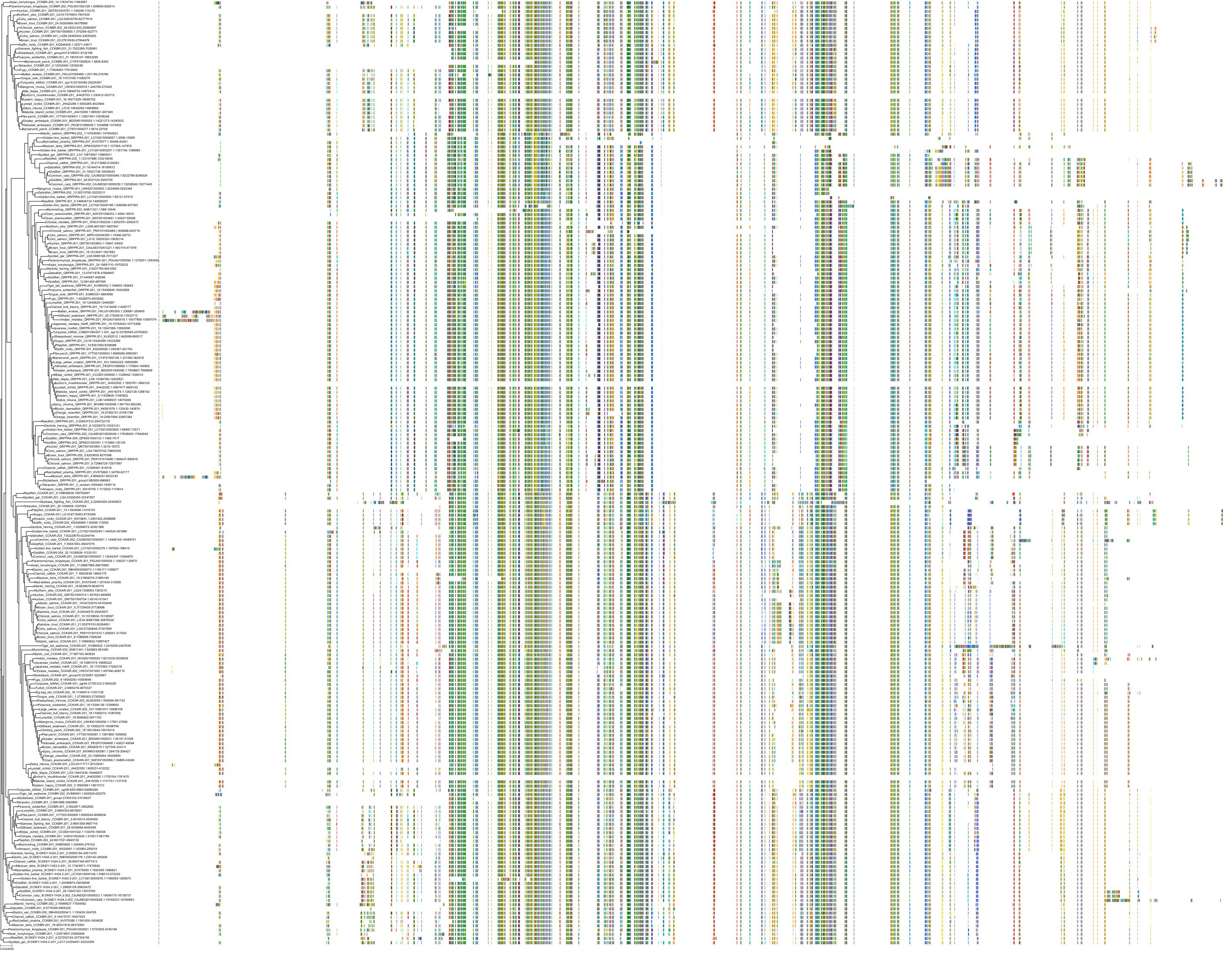
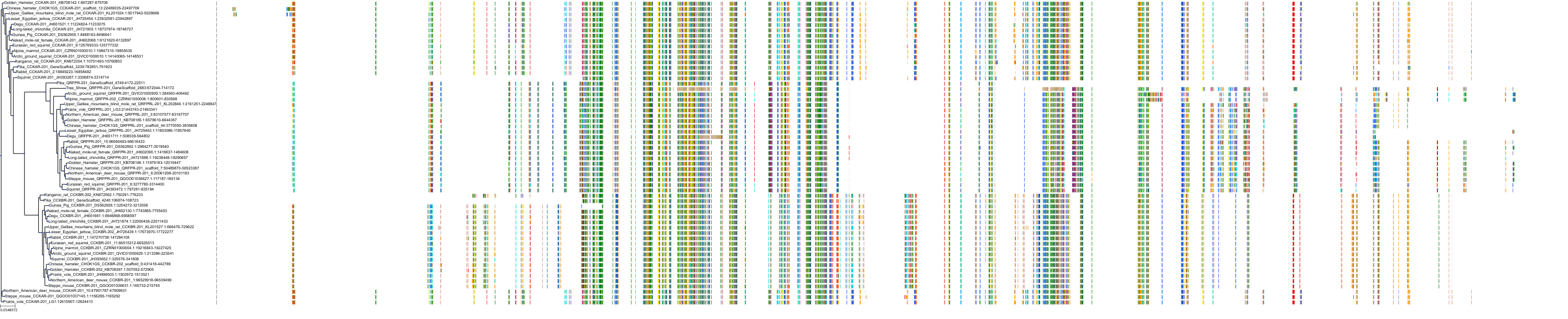
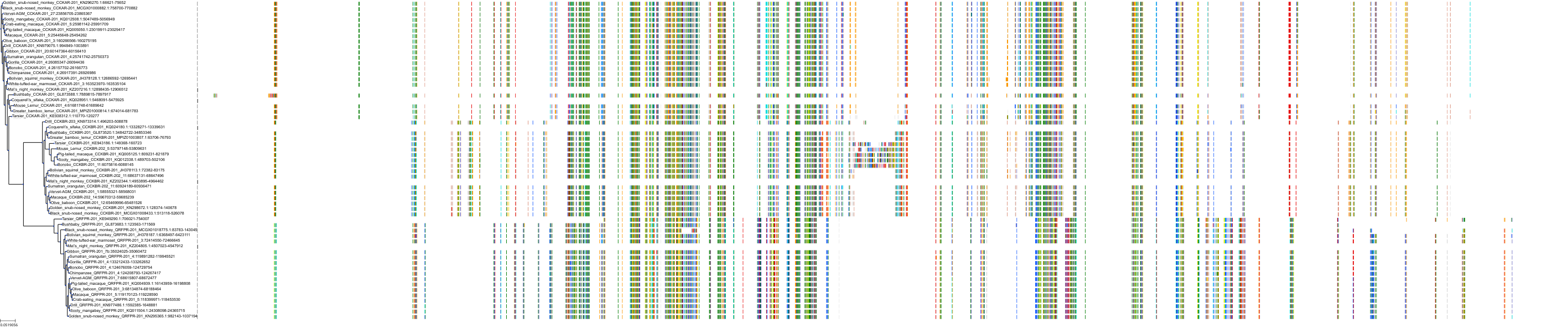
Target Conservation
|
Protein: Cholecystokinin A receptor Description: Cholecystokinin receptor type A Organism : Homo sapiens P32238 ENSG00000163394 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 135747 |
| ChEMBL | CHEMBL550781 |
| DrugBank | DB04856 |
| DrugCentral | 834 |
| FDA SRS | 69DY40RH9B |
| Guide to Pharmacology | 889 |
| PubChem | 65937 |
| SureChEMBL | SCHEMBL366142 |
| ZINC | ZINC000003801027 |