| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | G04BX14 |
| UNII | GB2433A4M3 |
| EPA CompTox | DTXSID0057627 |
Structure
| InChI Key | USRHYDPUVLEVMC-FQEVSTJZSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C21H23NO |
| Molecular Weight | 305.42 |
| AlogP | 4.91 |
| Hydrogen Bond Acceptor | 2.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 12.47 |
| Molecular species | BASE |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 23.0 |
Pharmacology
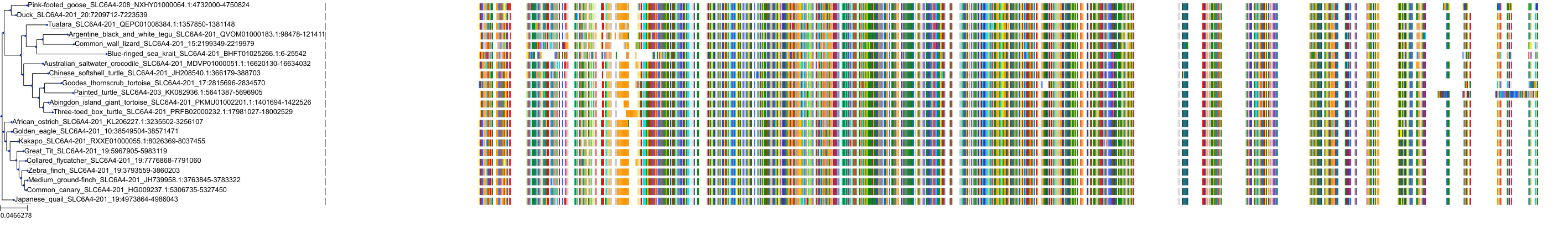
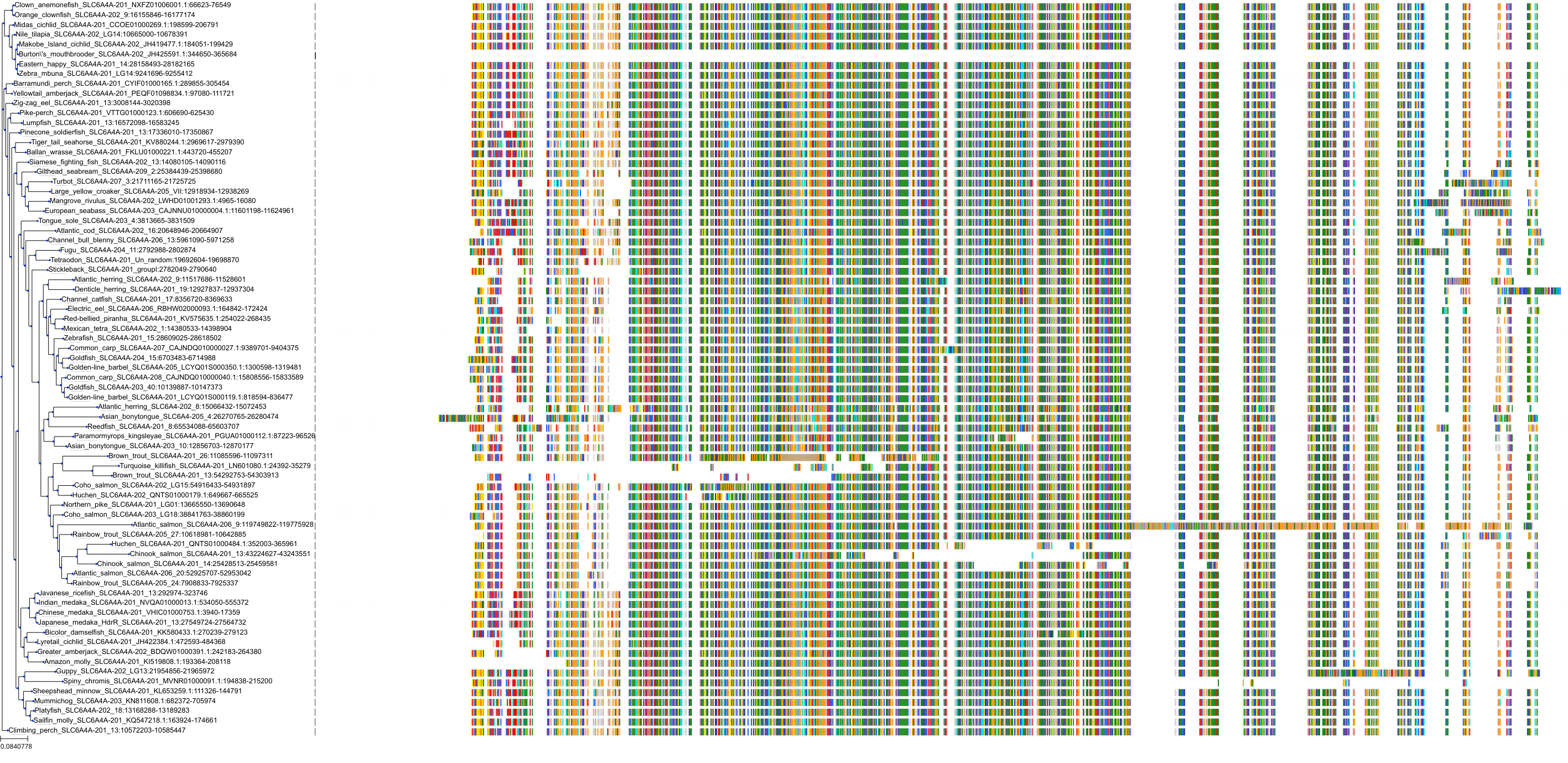
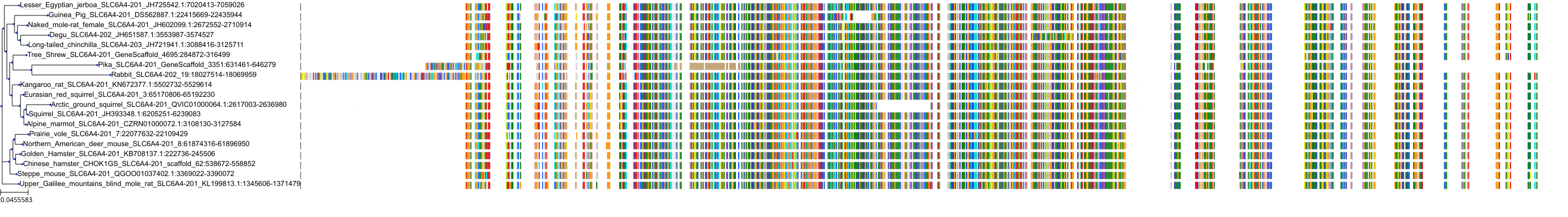
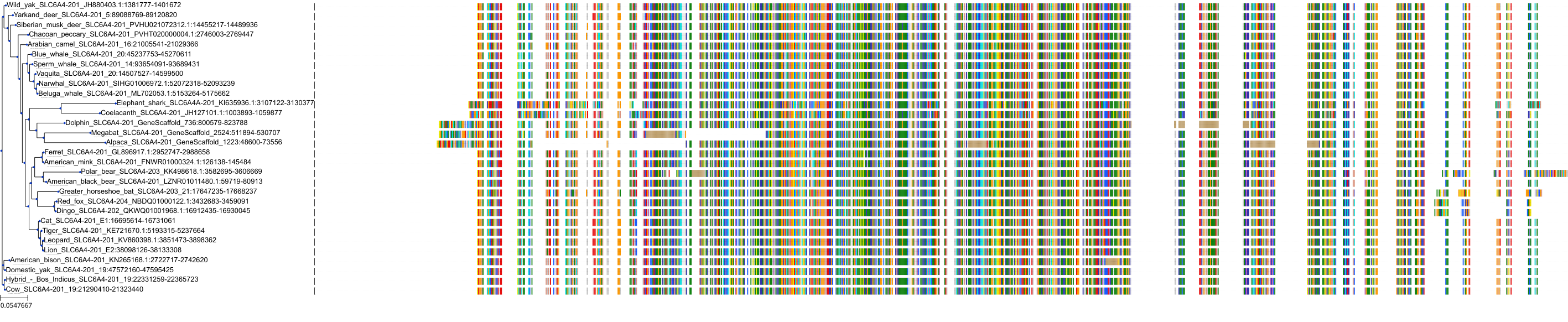
Target Conservation
|
Protein: Serotonin transporter Description: Sodium-dependent serotonin transporter Organism : Homo sapiens P31645 ENSG00000108576 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 135962 |
| ChEMBL | CHEMBL2110900 |
| DrugBank | DB04884 |
| DrugCentral | 4381 |
| FDA SRS | GB2433A4M3 |
| Guide to Pharmacology | 7901 |
| PharmGKB | PA166151992 |
| PubChem | 71353 |
| SureChEMBL | SCHEMBL34479 |
| ZINC | ZINC000001482019 |