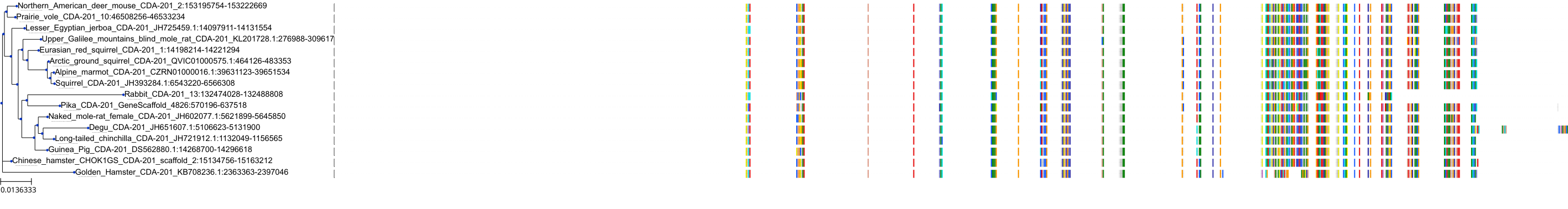Structure
| InChI Key | VUDZSIYXZUYWSC-DBRKOABJSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C9H14F2N2O5 |
| Molecular Weight | 268.22 |
| AlogP | -1.57 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 4.0 |
| Number of Rotational Bond | 2.0 |
| Polar Surface Area | 102.26 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 0.0 |
| Heavy Atoms | 18.0 |
Pharmacology
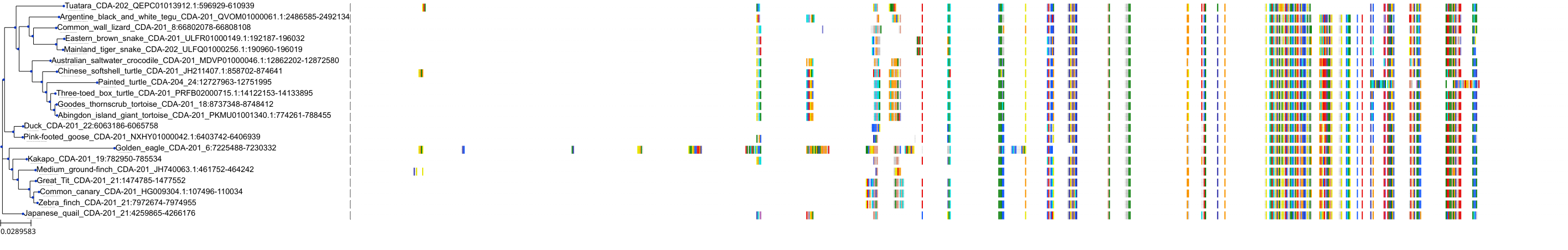
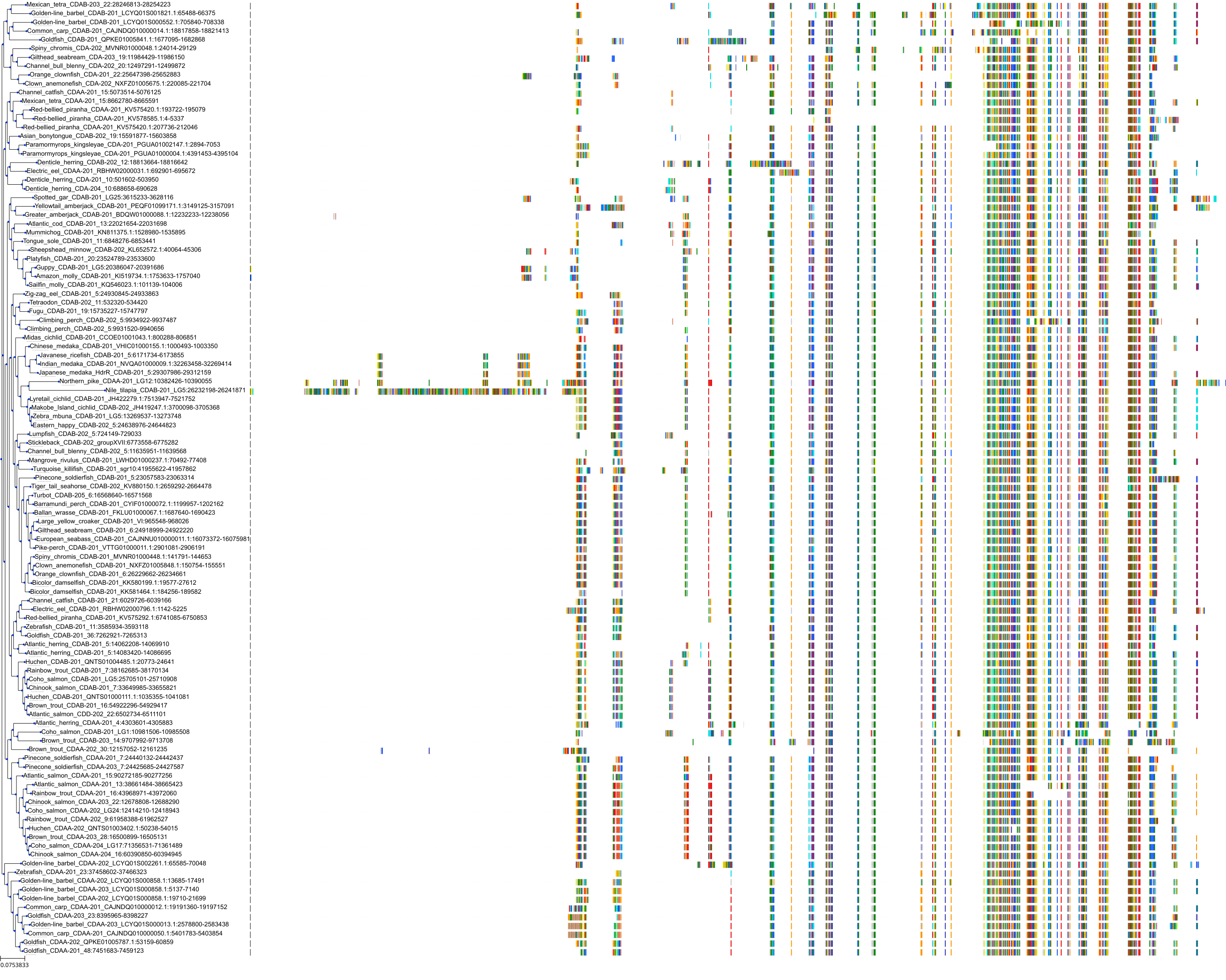
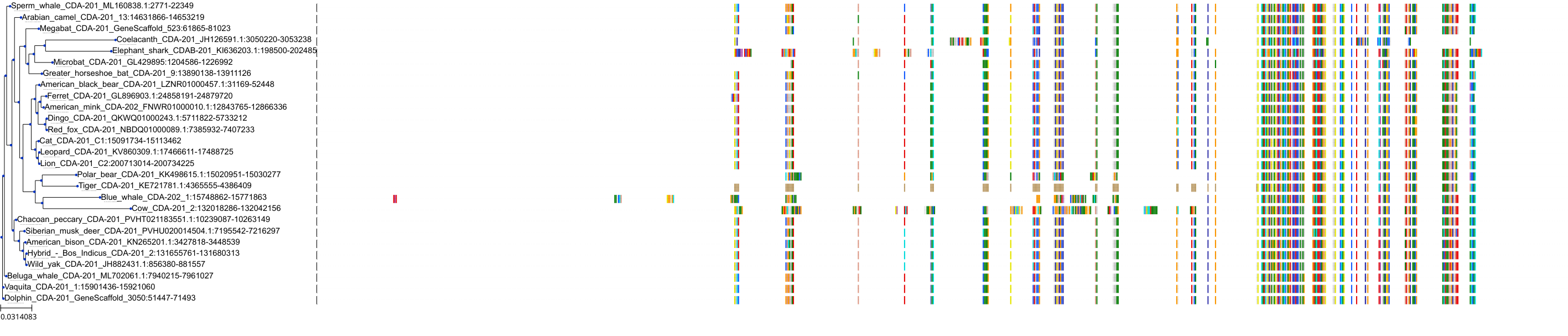
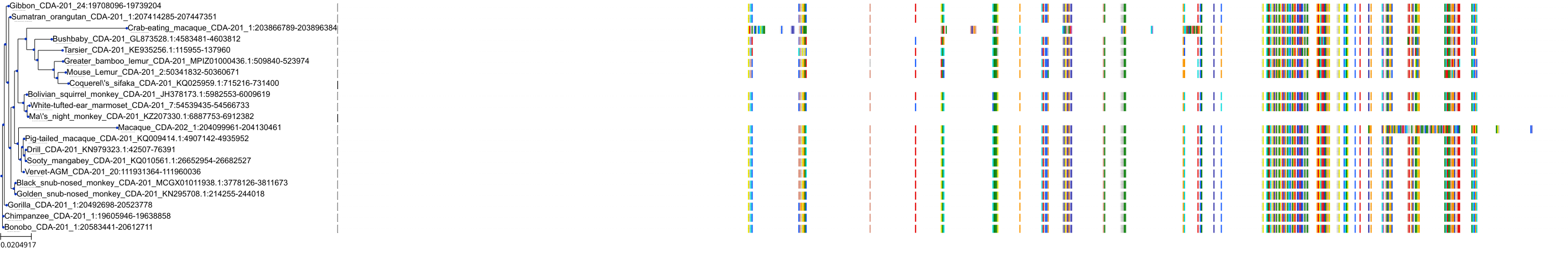
Target Conservation
|
Protein: Cytidine deaminase Description: Cytidine deaminase Organism : Homo sapiens P32320 ENSG00000158825 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3237547 |
| DrugBank | DB15694 |
| FDA SRS | 39IS23Q1EW |
| Guide to Pharmacology | 11101 |
| PubChem | 25267009 |
| SureChEMBL | SCHEMBL172256 |
| ZINC | ZINC000043205136 |
 Homo sapiens
Homo sapiens