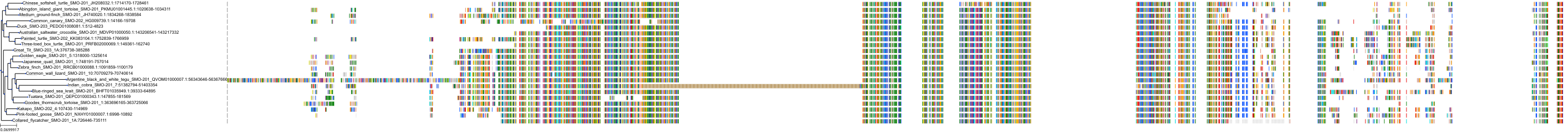Structure
| InChI Key | KLRRGBHZCJLIEL-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C30H27N5O |
| Molecular Weight | 473.58 |
| AlogP | 6.32 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 78.94 |
| Molecular species | BASE |
| Aromatic Rings | 5.0 |
| Heavy Atoms | 36.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Smoothened homolog antagonist | ANTAGONIST | PubMed |
Target Conservation
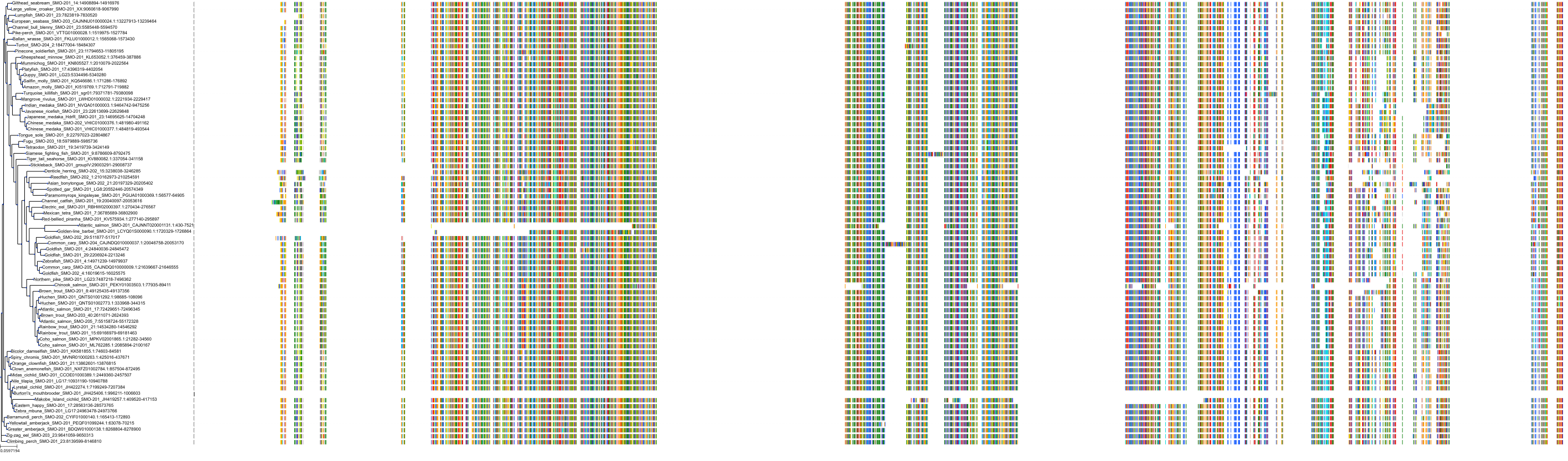
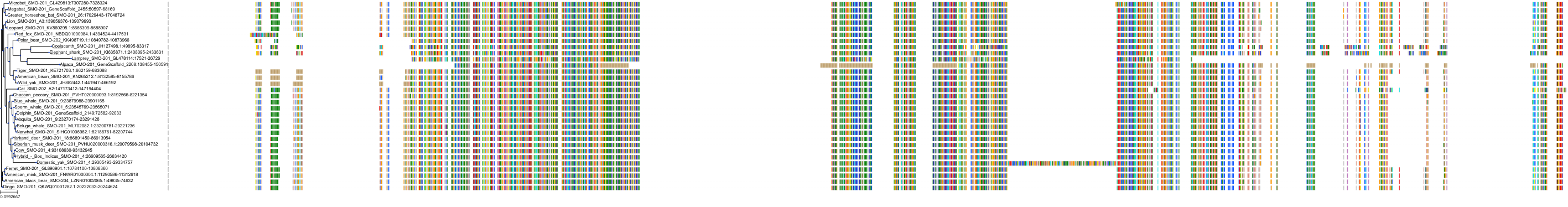
|
Protein: Smoothened homolog Description: Smoothened homolog Organism : Homo sapiens Q99835 ENSG00000128602 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3545403 |
| FDA SRS | 41J7ZJ239R |
| Guide to Pharmacology | 8202 |
| SureChEMBL | SCHEMBL4138073 |
| ZINC | ZINC000096170449 |