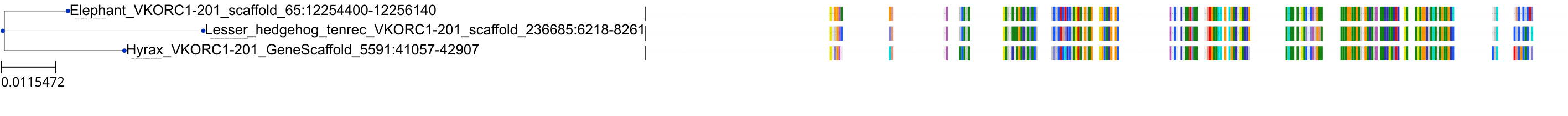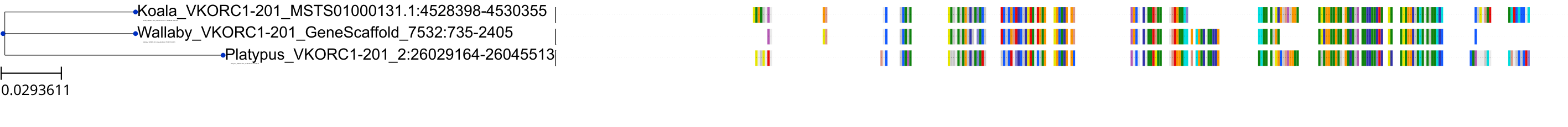| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | B01AA07 |
| UNII | I6WP63U32H |
| EPA CompTox | DTXSID2022541 |
Structure
| InChI Key | VABCILAOYCMVPS-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C19H15NO6 |
| Molecular Weight | 353.33 |
| AlogP | 3.52 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 110.65 |
| Molecular species | ACID |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 26.0 |
Pharmacology
Target Conservation
|
Protein: Vitamin k epoxide reductase complex subunit 1 isoform 1 Description: Vitamin K epoxide reductase complex subunit 1 Organism : Homo sapiens Q9BQB6 ENSG00000167397 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 53766 |
| ChEMBL | CHEMBL397420 |
| DrugBank | DB01418 |
| DrugCentral | 48 |
| FDA SRS | I6WP63U32H |
| Human Metabolome Database | HMDB0015487 |
| Guide to Pharmacology | 9015 |
| PubChem | 54676537 |
| SureChEMBL | SCHEMBL33543 |
 Rattus norvegicus
Rattus norvegicus