| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Salt-form |
| UNII | FWV8GJ56ZN |
| EPA CompTox | DTXSID6026248 |
Structure
| InChI Key | FSSICIQKZGUEAE-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C16H22ClN3 |
| Molecular Weight | 291.83 |
| AlogP | 2.65 |
| Hydrogen Bond Acceptor | 3.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 19.37 |
| Molecular species | BASE |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 19.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Histamine H1 receptor antagonist | ANTAGONIST | PubMed |
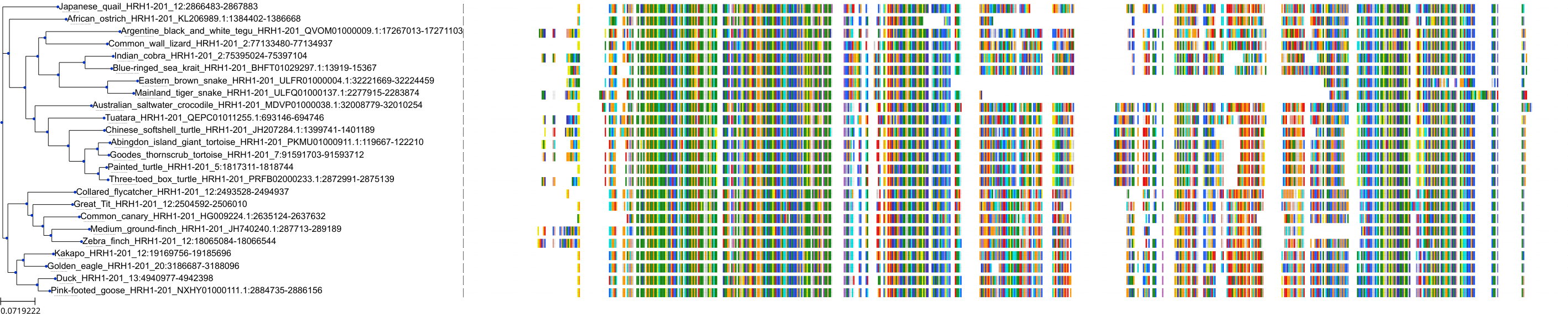
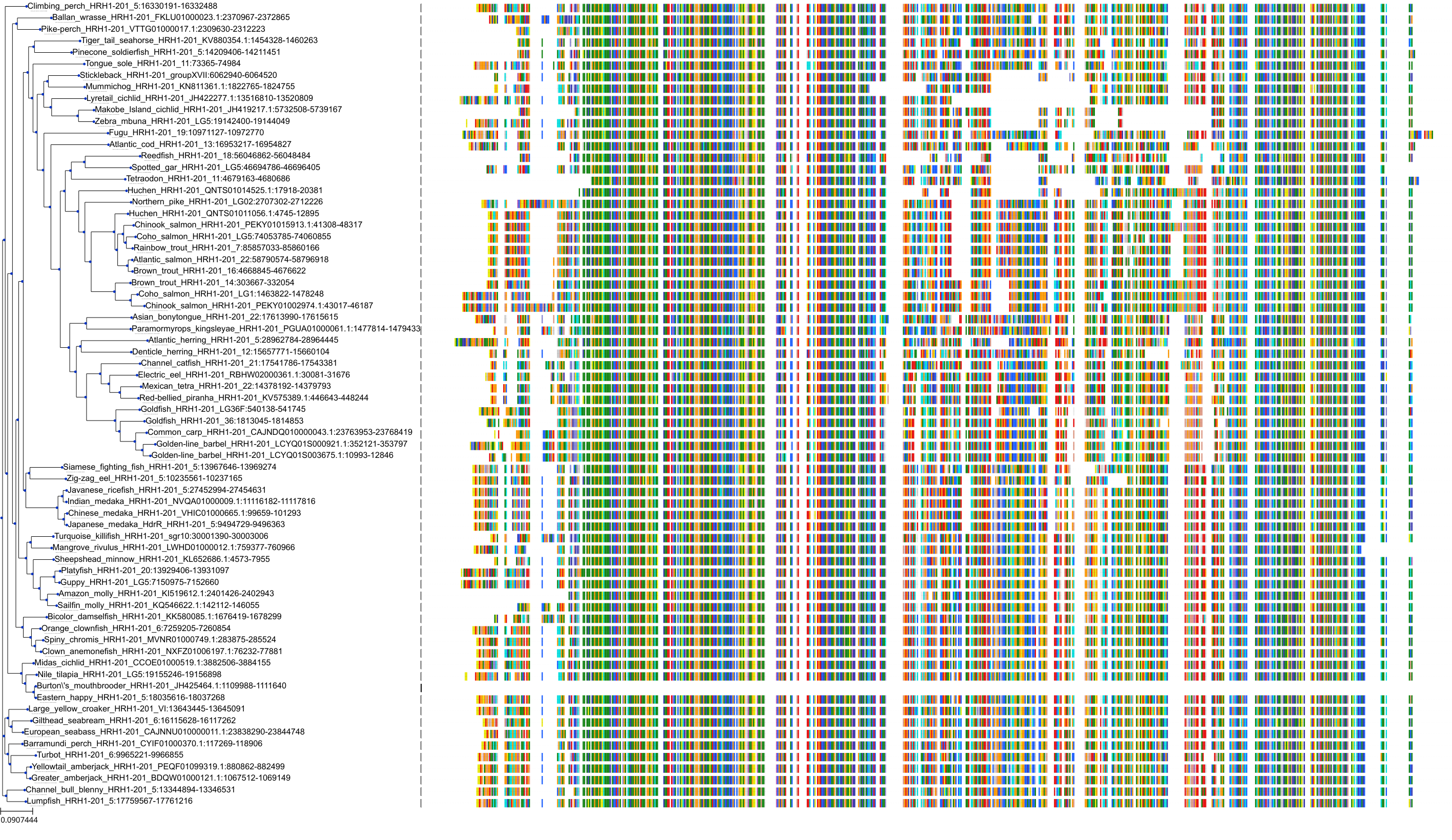
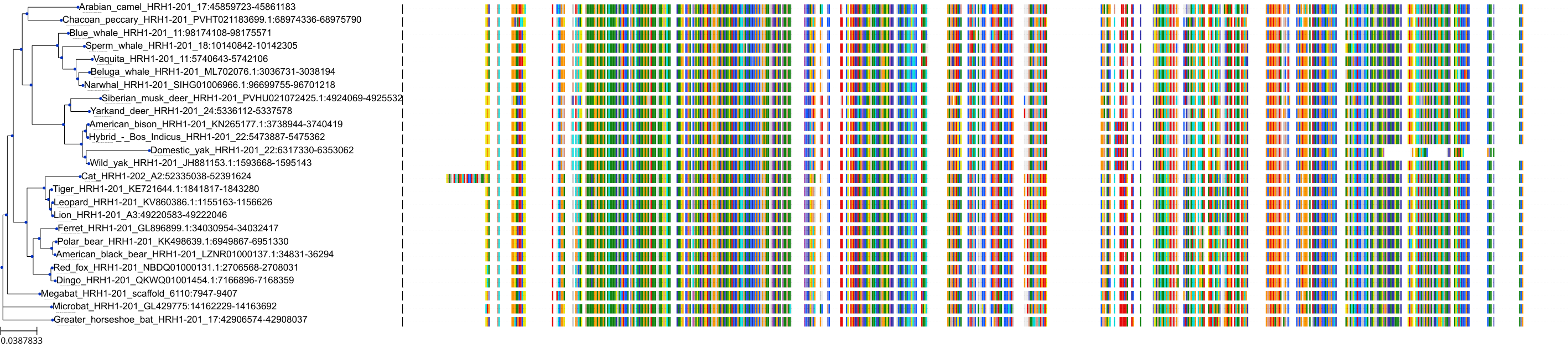
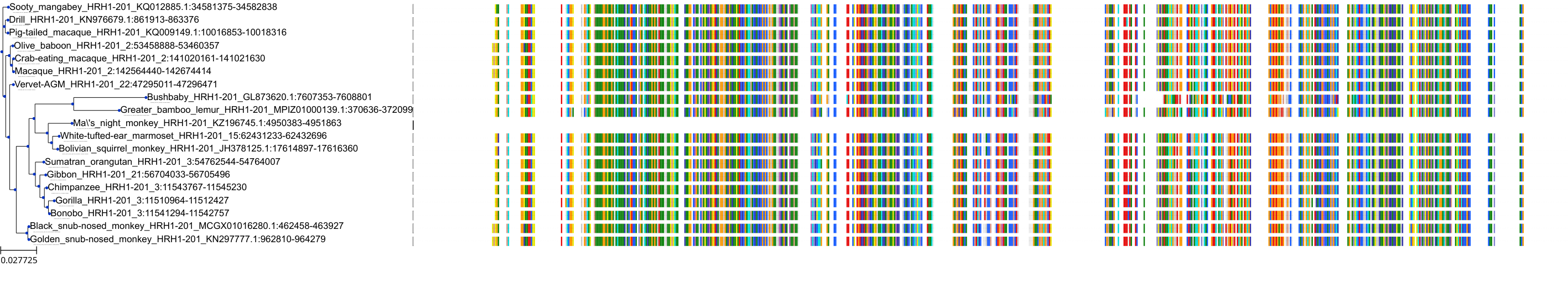
Target Conservation
|
Protein: Histamine H1 receptor Description: Histamine H1 receptor Organism : Homo sapiens P35367 ENSG00000196639 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL1200446 |
| FDA SRS | FWV8GJ56ZN |
| Guide to Pharmacology | 7318 |
| KEGG | C07180 |
| PubChem | 9066 |
| SureChEMBL | SCHEMBL98916 |
| ZINC | ZINC00057524 |