| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Salt-form |
| UNII | BW9B0ZE037 |
| EPA CompTox | DTXSID4046076 |
| Parent Compound: | SILDENAFIL |
Structure
| InChI Key | DEIYFTQMQPDXOT-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C28H38N6O11S |
| Molecular Weight | 666.71 |
| AlogP | 1.61 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 113.42 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 33.0 |
Metabolites Network
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Phosphodiesterase 5A inhibitor | INHIBITOR | DailyMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Phosphodiesterase
Phosphodiesterase 5
Phosphodiesterase 5A
|
- | 2.2-5.1 | - | - | 13-91 | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 6
Phosphodiesterase 6C
|
- | - | - | - | 8-85 |
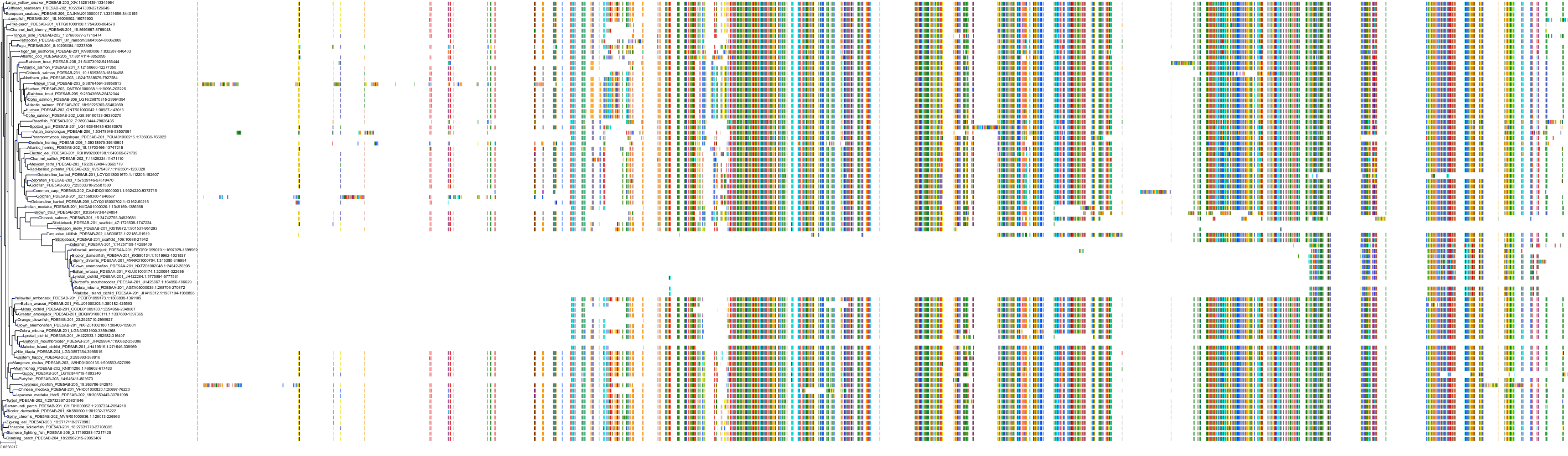
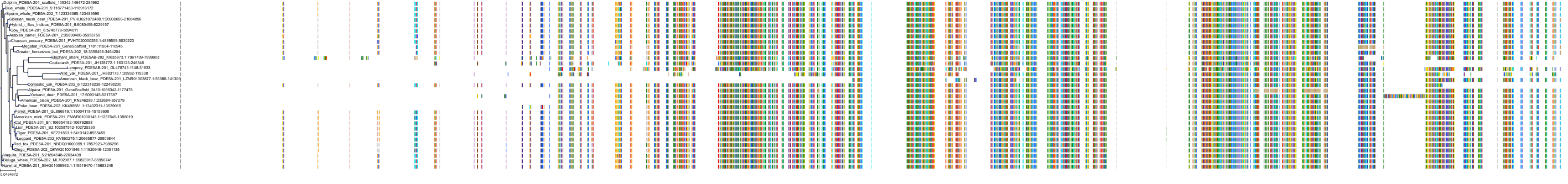
Target Conservation
|
Protein: Phosphodiesterase 5A Description: cGMP-specific 3',5'-cyclic phosphodiesterase Organism : Homo sapiens O76074 ENSG00000138735 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 58987 |
| ChEMBL | CHEMBL1737 |
| FDA SRS | BW9B0ZE037 |
| Guide to Pharmacology | 4743 |
| KEGG | C07259 |
| PDB | VIA |
| PubChem | 135413523 |
| SureChEMBL | SCHEMBL33982 |
| ZINC | ZINC19796168 |
 Homo sapiens
Homo sapiens
 Sus scrofa
Sus scrofa