| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L01XX62 |
| UNII | Q2PCN8MAM6 |
Structure
| InChI Key | WIJZXSAJMHAVGX-DHLKQENFSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C28H22ClF3N6O3 |
| Molecular Weight | 582.97 |
| AlogP | 4.32 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 119.29 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 41.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Isocitrate dehydrogenase [NADP] cytoplasmic inhibitor | INHIBITOR | FDA |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Oxidoreductase
|
12.47-27.23 | 3.5-65 | - | - | - |
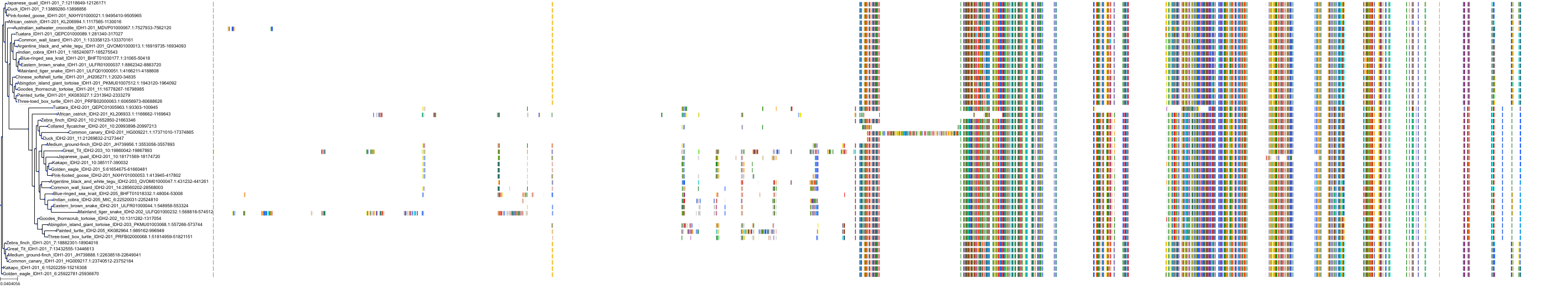
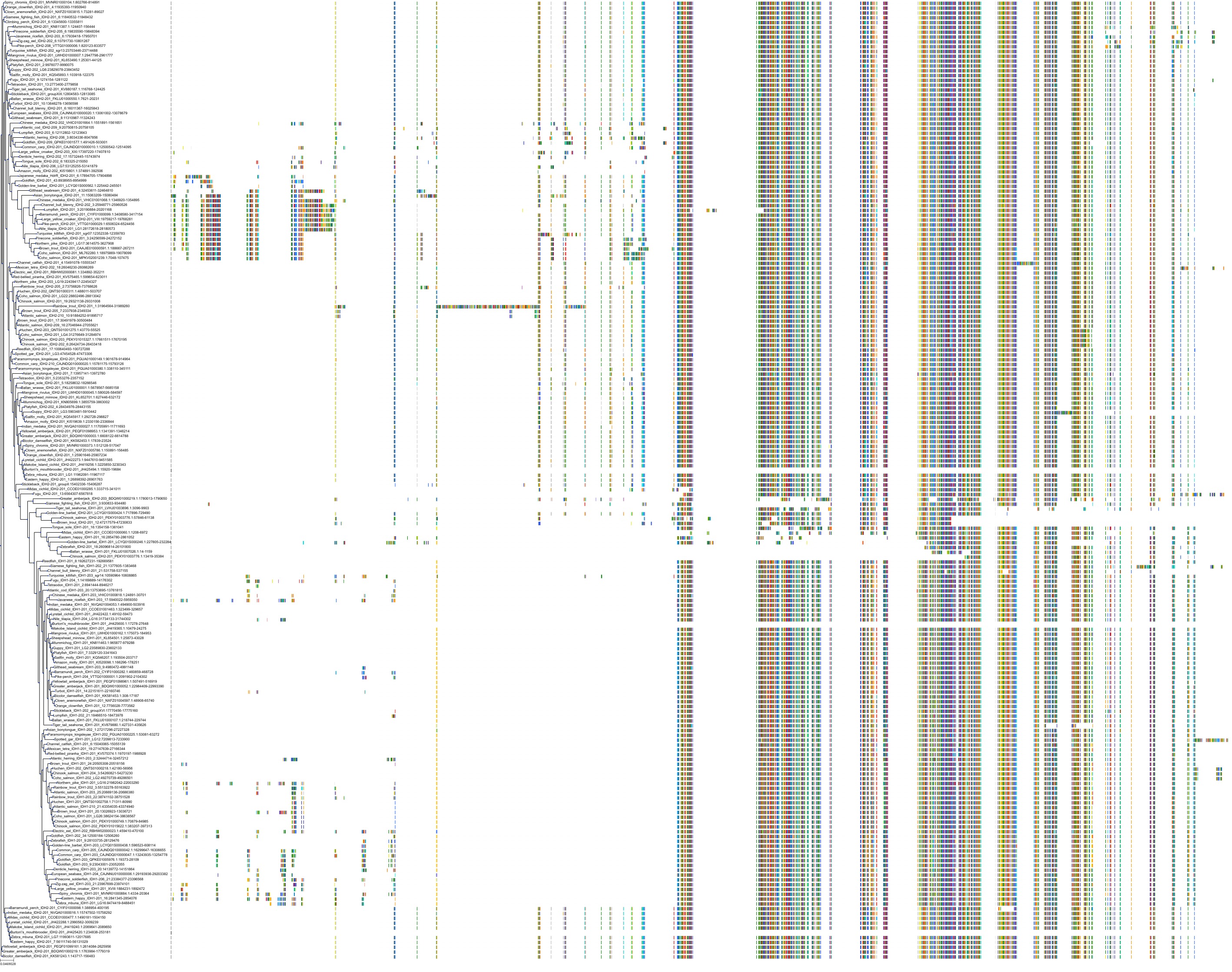
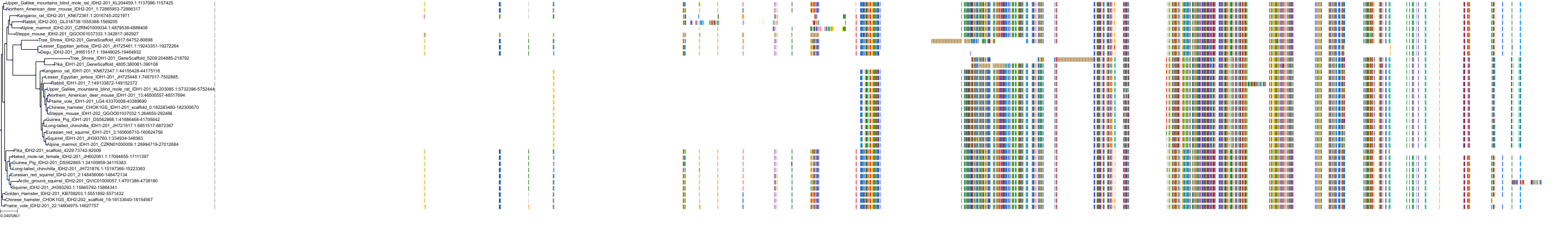
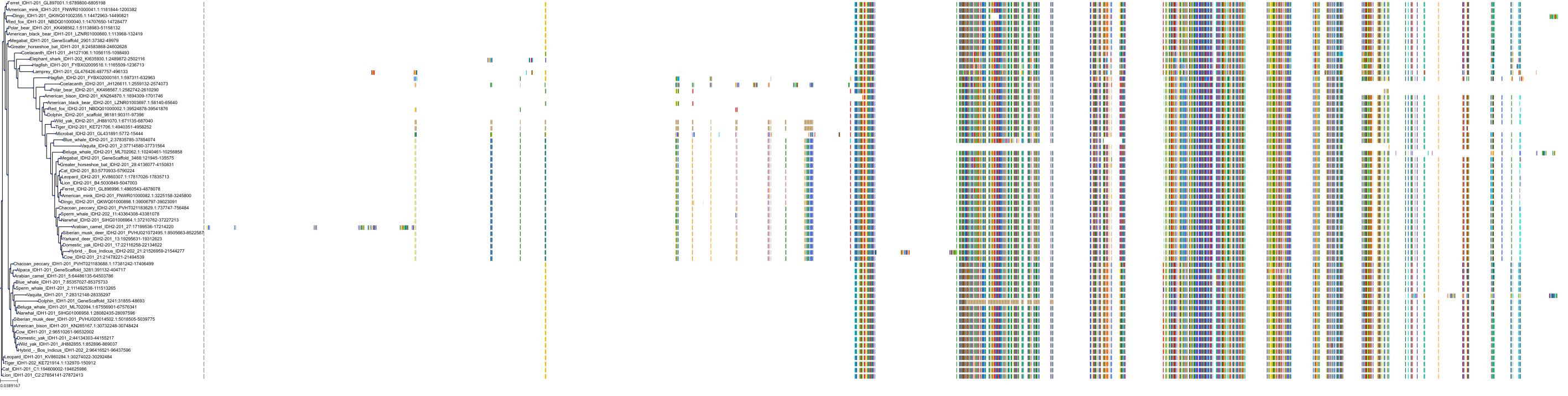
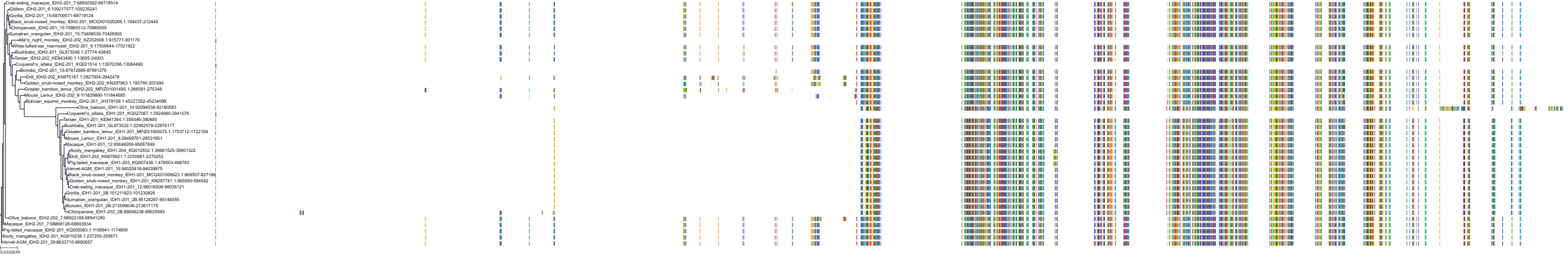
Target Conservation
|
Protein: Isocitrate dehydrogenase [NADP] cytoplasmic Description: Isocitrate dehydrogenase [NADP] cytoplasmic Organism : Homo sapiens O75874 ENSG00000138413 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 145430 |
| ChEMBL | CHEMBL3989958 |
| DrugBank | DB14568 |
| DrugCentral | 5292 |
| FDA SRS | Q2PCN8MAM6 |
| Guide to Pharmacology | 9217 |
| PubChem | 71657455 |
| SureChEMBL | SCHEMBL15122512 |
| ZINC | ZINC000205136523 |
 Homo sapiens
Homo sapiens