| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Salt-form |
| UNII | 4K04IQ1OF4 |
| EPA CompTox | DTXSID3046617 |
| Parent Compound: | EPOPROSTENOL |
Structure
| InChI Key | LMHIPJMTZHDKEW-XQYLJSSYSA-M |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C20H31NaO5 |
| Molecular Weight | 374.45 |
| AlogP | 3.41 |
| Hydrogen Bond Acceptor | 4.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 10.0 |
| Polar Surface Area | 86.99 |
| Molecular species | ACID |
| Aromatic Rings | 0.0 |
| Heavy Atoms | 25.0 |
Pharmacology
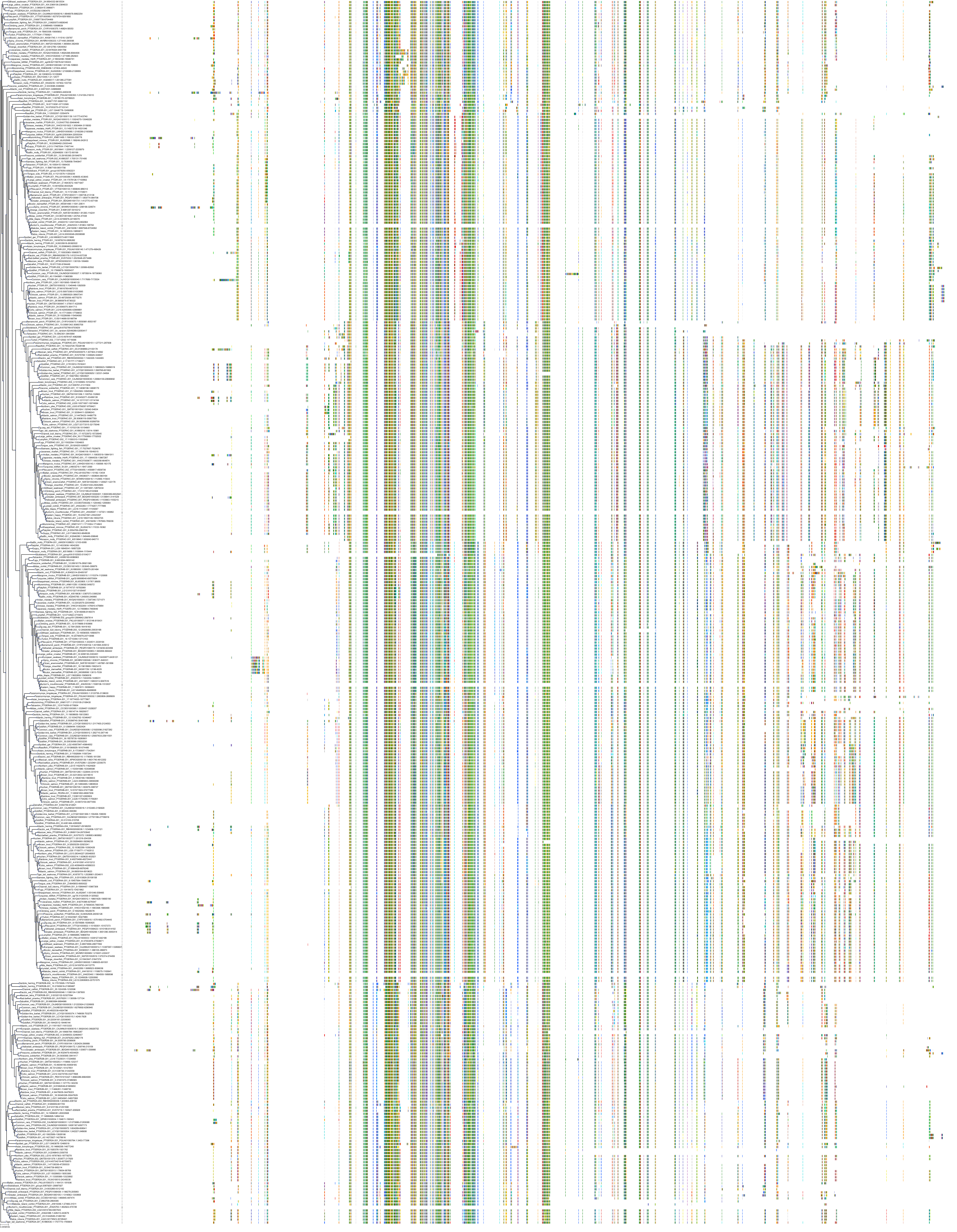
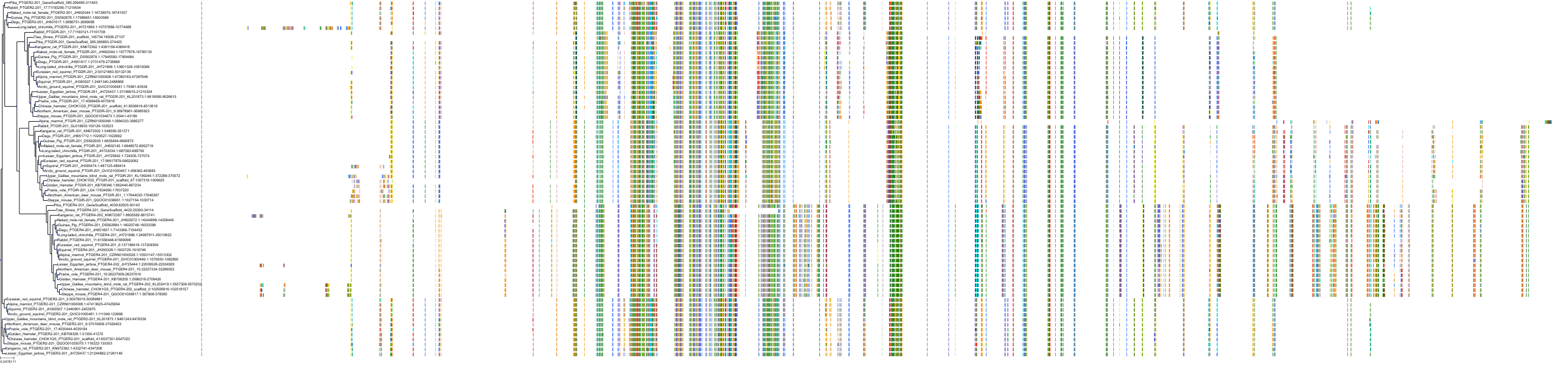
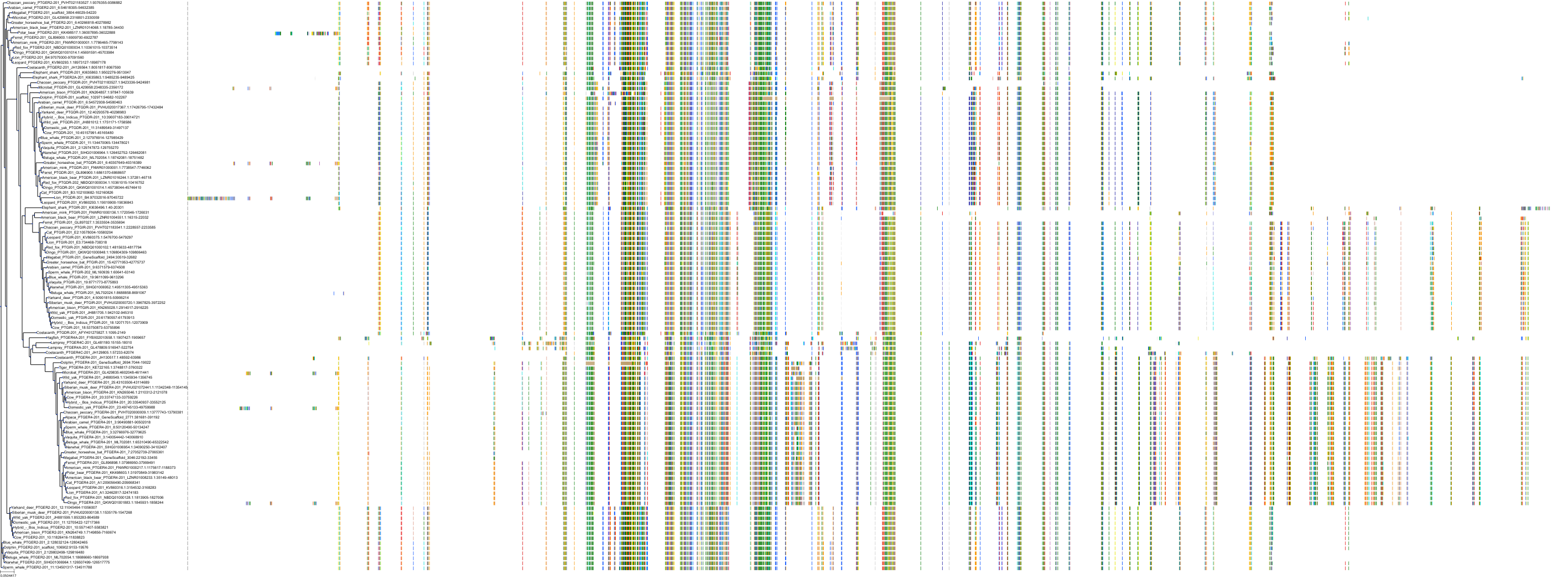
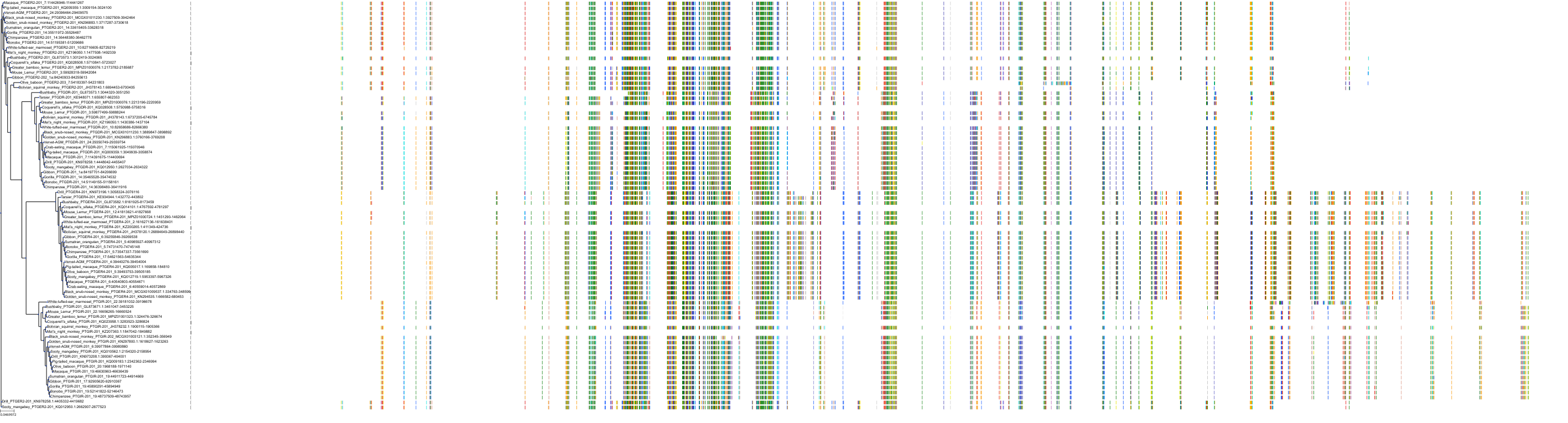
Target Conservation
|
Protein: Prostanoid IP receptor Description: Prostacyclin receptor Organism : Homo sapiens P43119 ENSG00000160013 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 31548 |
| ChEMBL | CHEMBL962 |
| FDA SRS | 4K04IQ1OF4 |
| PubChem | 6364626 |
| SureChEMBL | SCHEMBL41344 |
 Bos taurus
Bos taurus