| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Mixture |
| UNII | 4U07F515LG |
Structure
| InChI Key | PLILLUUXAVKBPY-SBIAVEDLSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C29H36N6O6 |
| Molecular Weight | 564.64 |
| AlogP | 4.56 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 114.59 |
| Molecular species | ACID |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 33.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Thrombopoietin receptor agonist | AGONIST | DailyMed |
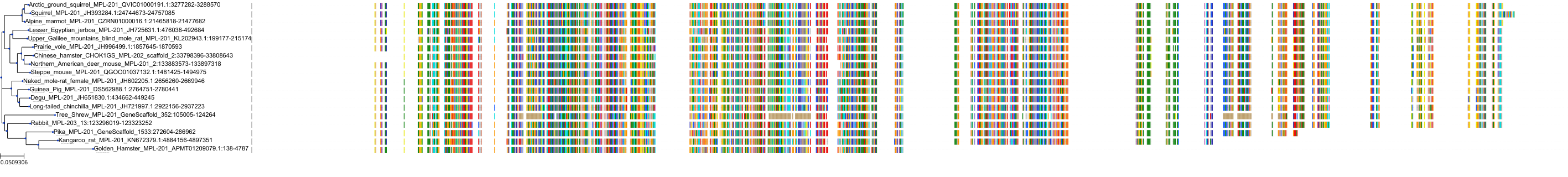
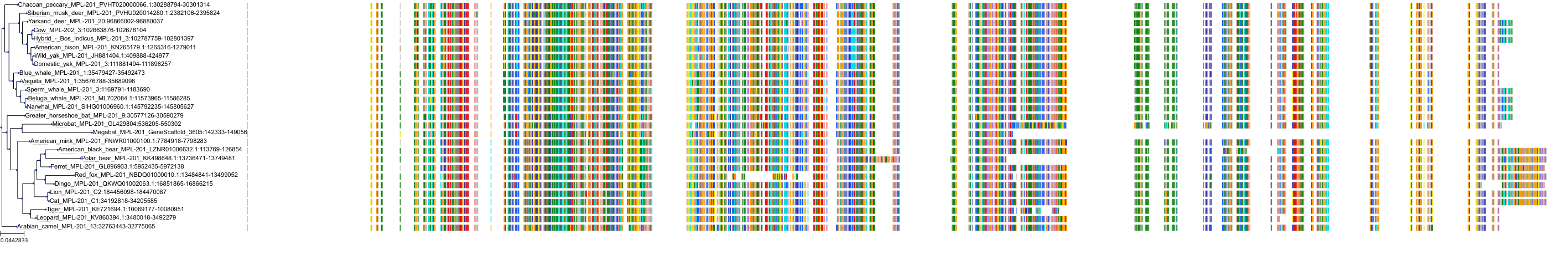
Target Conservation
|
Protein: Thrombopoietin receptor Description: Thrombopoietin receptor Organism : Homo sapiens P40238 ENSG00000117400 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3989691 |
| FDA SRS | 4U07F515LG |
| ZINC | ZINC11679756 |