| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | D07AB08 S01BA11 |
| UNII | J280872D1O |
| EPA CompTox | DTXSID7046756 |
Structure
| InChI Key | WBGKWQHBNHJJPZ-LECWWXJVSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C24H32O6 |
| Molecular Weight | 416.51 |
| AlogP | 2.33 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 2.0 |
| Polar Surface Area | 93.06 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 0.0 |
| Heavy Atoms | 30.0 |
Pharmacology
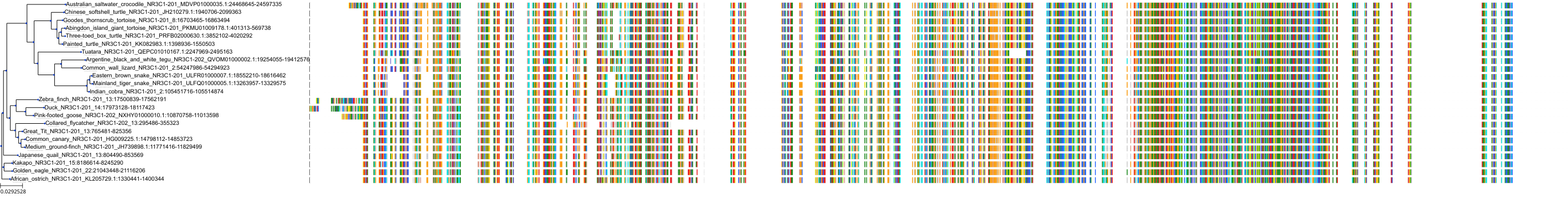
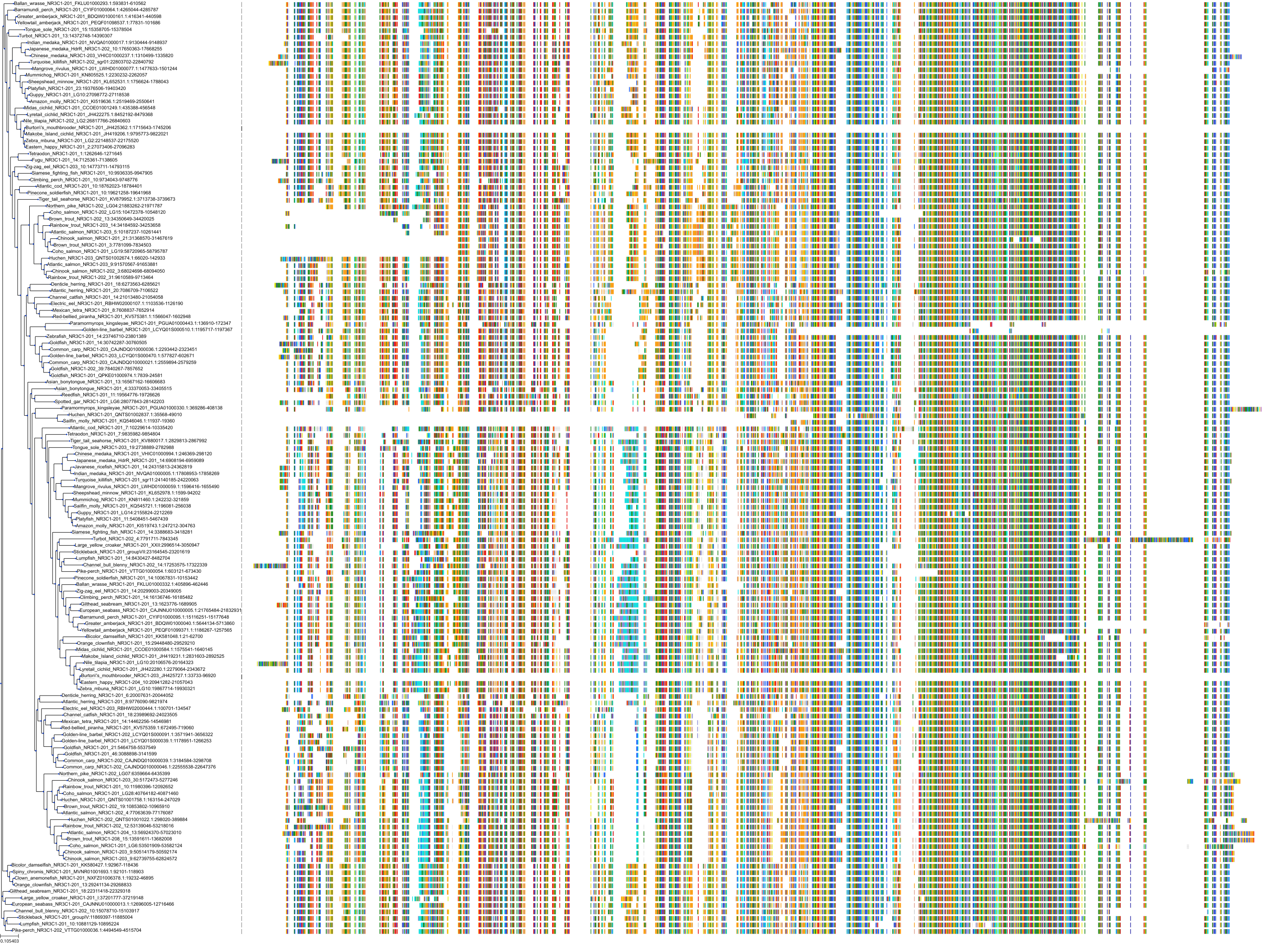
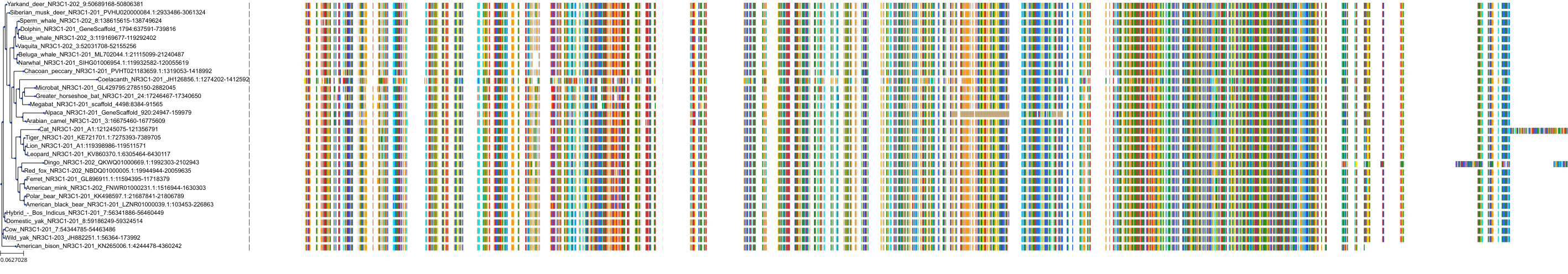
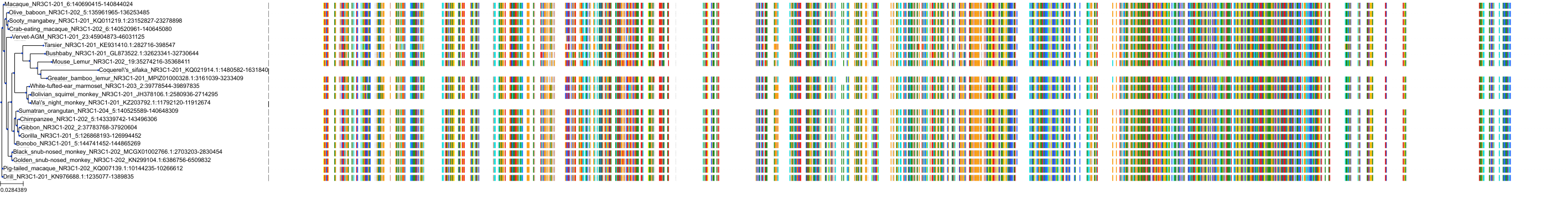
Target Conservation
|
Protein: Glucocorticoid receptor Description: Glucocorticoid receptor Organism : Homo sapiens P04150 ENSG00000113580 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 204734 |
| ChEMBL | CHEMBL1201109 |
| DrugBank | DB01260 |
| DrugCentral | 3135 |
| FDA SRS | J280872D1O |
| Human Metabolome Database | HMDB0015389 |
| Guide to Pharmacology | 7066 |
| PharmGKB | PA164776996 |
| PubChem | 5311066 |
| SureChEMBL | SCHEMBL3631 |
| ZINC | ZINC000004212851 |