| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | QBL8IZH14X |
| EPA CompTox | DTXSID0045460 |
Structure
| InChI Key | SXYZQZLHAIHKKY-GSTUPEFVSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C27H36ClFO5 |
| Molecular Weight | 495.03 |
| AlogP | 4.6 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 3.0 |
| Polar Surface Area | 80.67 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 0.0 |
| Heavy Atoms | 34.0 |
Pharmacology
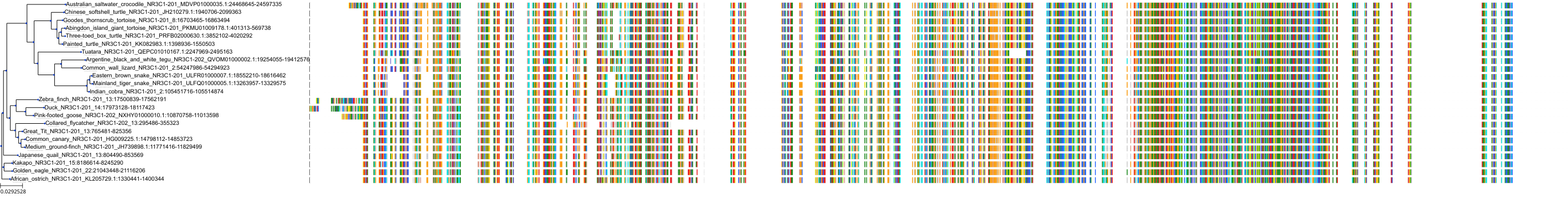
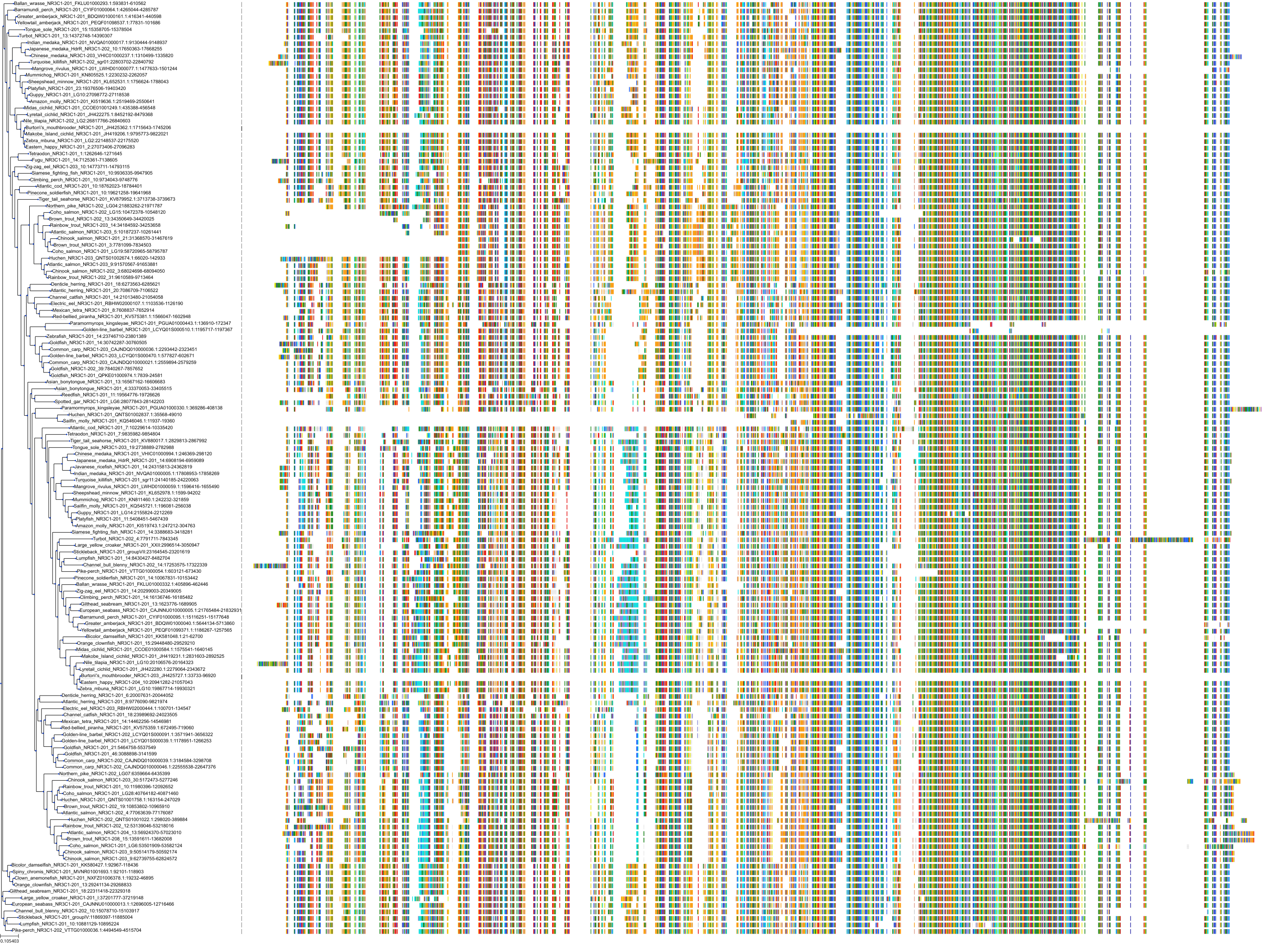
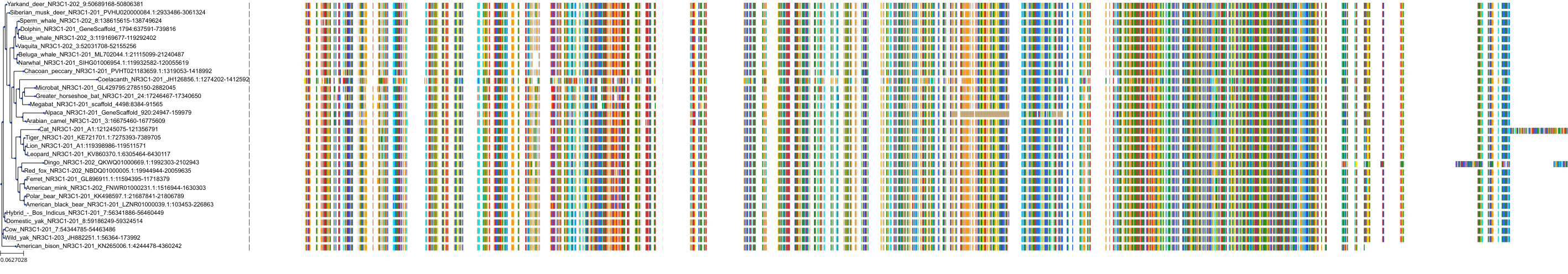
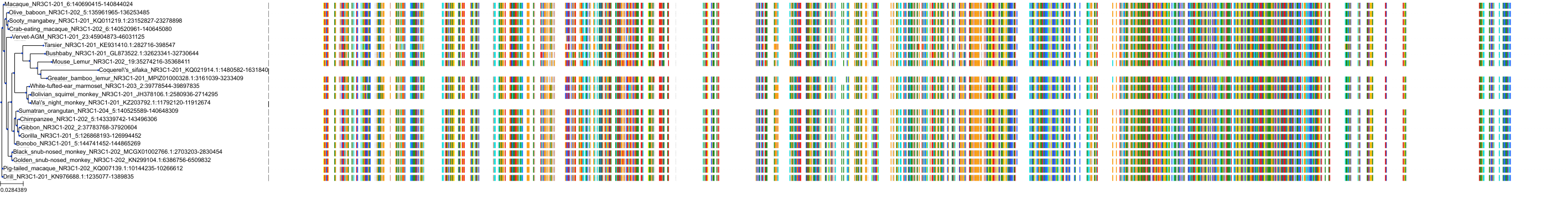
Target Conservation
|
Protein: Glucocorticoid receptor Description: Glucocorticoid receptor Organism : Homo sapiens P04150 ENSG00000113580 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 59583 |
| ChEMBL | CHEMBL1200975 |
| DrugCentral | 4606 |
| FDA SRS | QBL8IZH14X |
| PharmGKB | PA164744013 |
| PubChem | 5282493 |
| SureChEMBL | SCHEMBL5120 |
| ZINC | ZINC000004212603 |