| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | B01AC23 |
| UNII | N7Z035406B |
| EPA CompTox | DTXSID9045132 |
Structure
| InChI Key | RRGUKTPIGVIEKM-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C20H27N5O2 |
| Molecular Weight | 369.47 |
| AlogP | 3.46 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 81.93 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 27.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Phosphodiesterase 3A inhibitor | INHIBITOR | DailyMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Phosphodiesterase
Phosphodiesterase 3
Phosphodiesterase 3A
|
- | 3.89-840 | - | - | - | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 3
Phosphodiesterase 3B
|
- | 190-840 | - | - | - | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC21/SLCO family of organic anion transporting polypeptides
|
- | - | - | - | 98.73-207.15 |
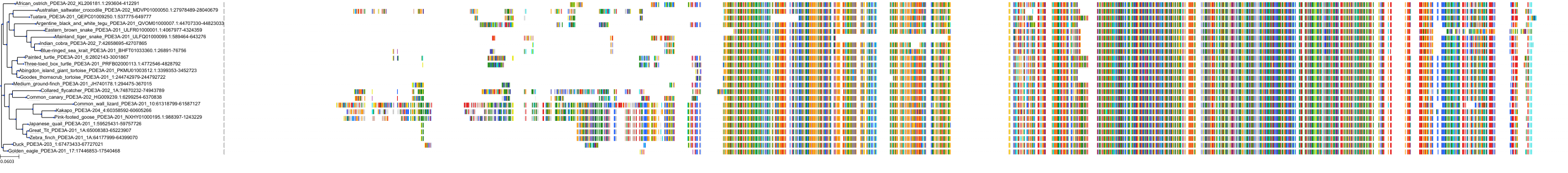
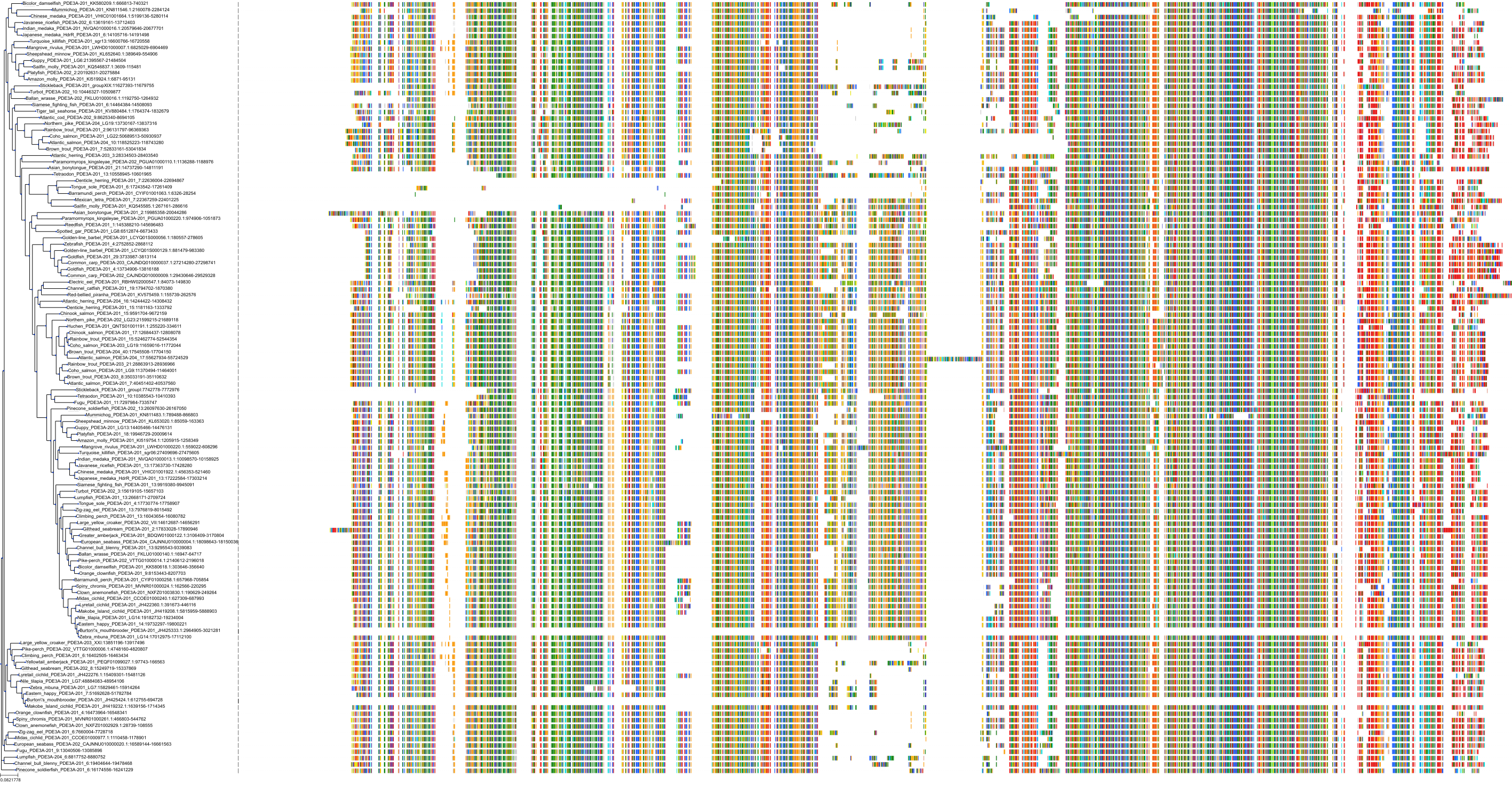
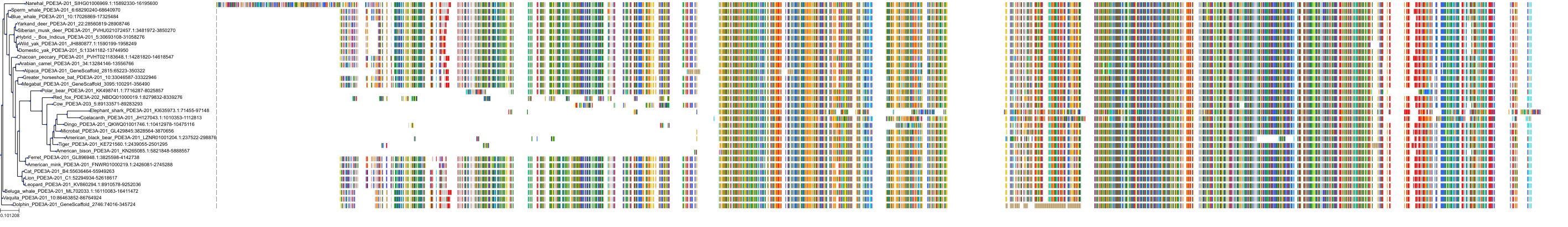
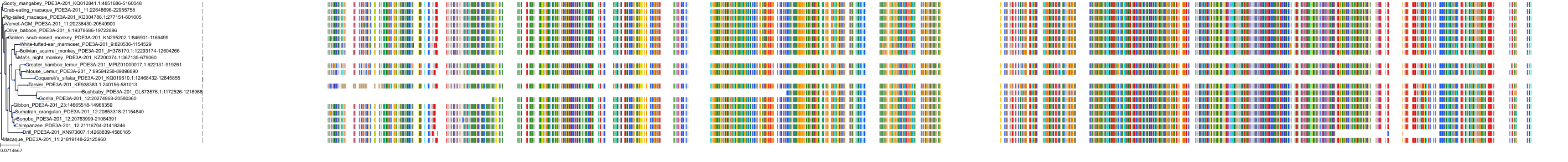
Target Conservation
|
Protein: Phosphodiesterase 3A Description: cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A Organism : Homo sapiens Q14432 ENSG00000172572 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 31401 |
| ChEMBL | CHEMBL799 |
| DrugBank | DB01166 |
| DrugCentral | 644 |
| FDA SRS | N7Z035406B |
| Human Metabolome Database | HMDB0015297 |
| Guide to Pharmacology | 7148 |
| PharmGKB | PA164746334 |
| PubChem | 2754 |
| SureChEMBL | SCHEMBL16128 |
| ZINC | ZINC000001552174 |
 Cricetulus griseus
Cricetulus griseus
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Rattus norvegicus
Rattus norvegicus