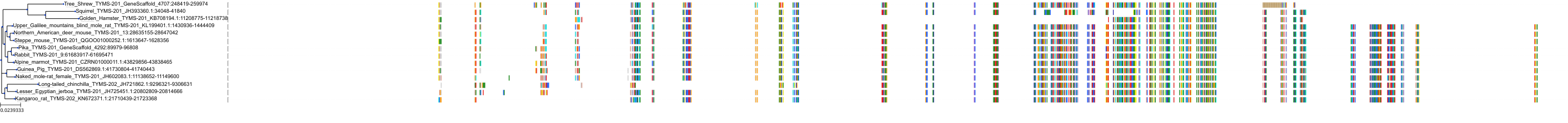| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L01BC06 |
| UNII | 6804DJ8Z9U |
| EPA CompTox | DTXSID3046451 |
Structure
| InChI Key | GAGWJHPBXLXJQN-UORFTKCHSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C15H22FN3O6 |
| Molecular Weight | 359.35 |
| AlogP | 0.76 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 122.91 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 25.0 |
Pharmacology
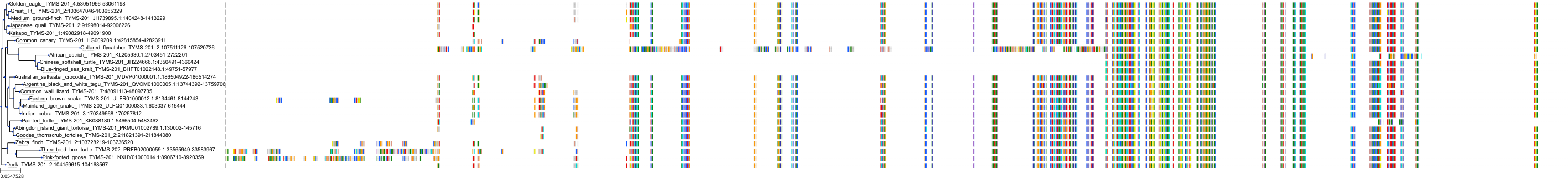
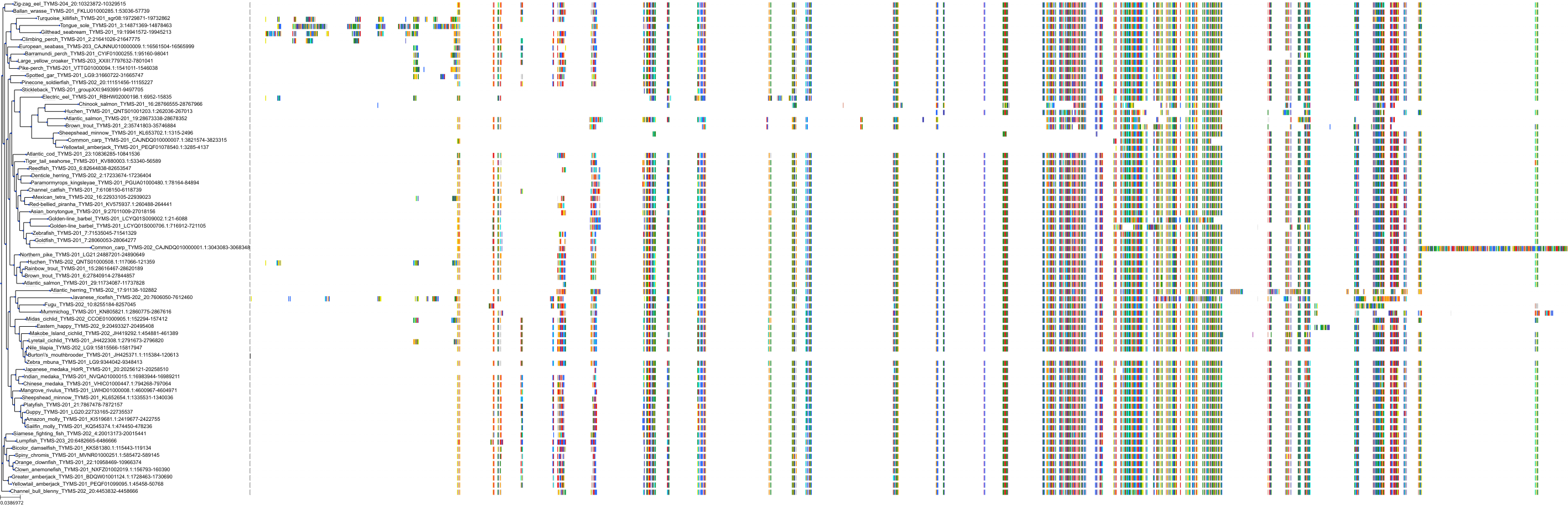
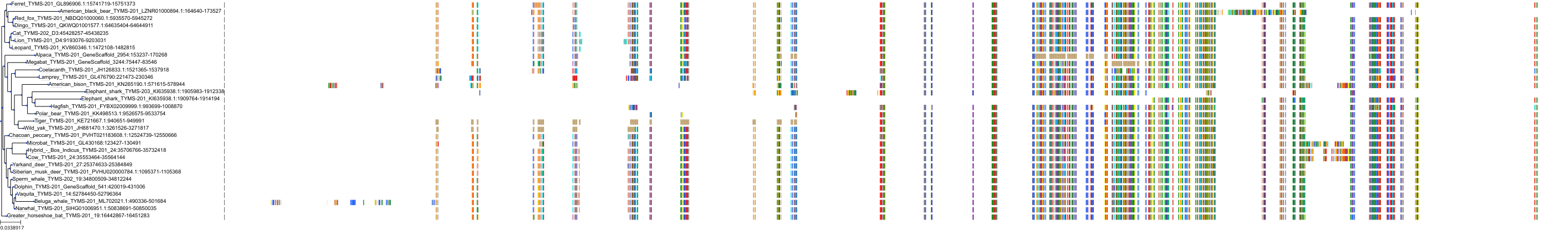
Target Conservation
|
Protein: Thymidylate synthase Description: Thymidylate synthase Organism : Homo sapiens P04818 ENSG00000176890 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 31348 |
| ChEMBL | CHEMBL1773 |
| DrugBank | DB01101 |
| DrugCentral | 480 |
| FDA SRS | 6804DJ8Z9U |
| Human Metabolome Database | HMDB0015233 |
| Guide to Pharmacology | 6799 |
| KEGG | C12650 |
| PharmGKB | PA448771 |
| PubChem | 60953 |
| SureChEMBL | SCHEMBL8153 |
| ZINC | ZINC000003806413 |
 Homo sapiens
Homo sapiens