| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Mixture |
| UNII | V1T5K7RZ0B |
Structure
| InChI Key | USNRYVNRPYXCSP-JUGPPOIOSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C32H33ClFN5O11 |
| Molecular Weight | 718.09 |
| AlogP | 4.39 |
| Hydrogen Bond Acceptor | 7.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 8.0 |
| Polar Surface Area | 88.61 |
| Molecular species | BASE |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 34.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Epidermal growth factor receptor erbB1 inhibitor | INHIBITOR | DailyMed |
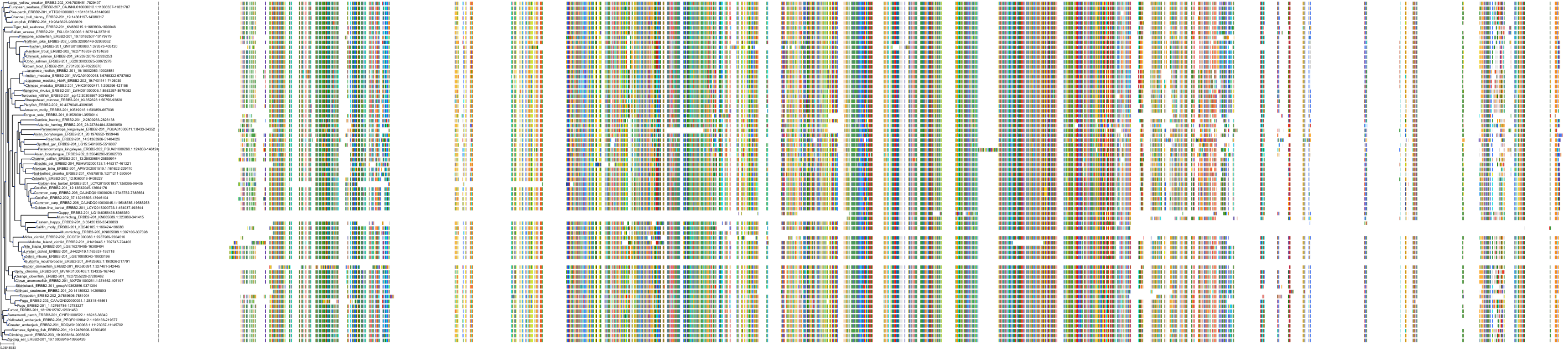
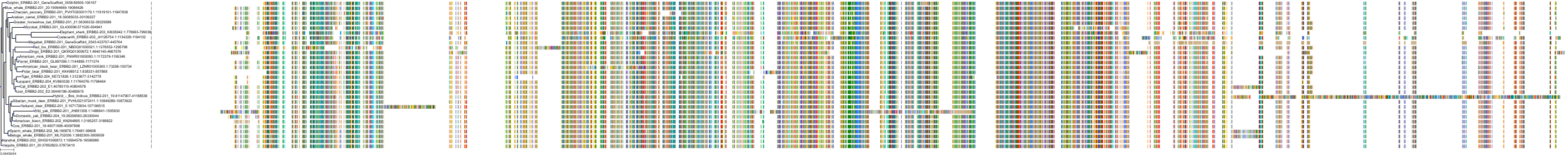
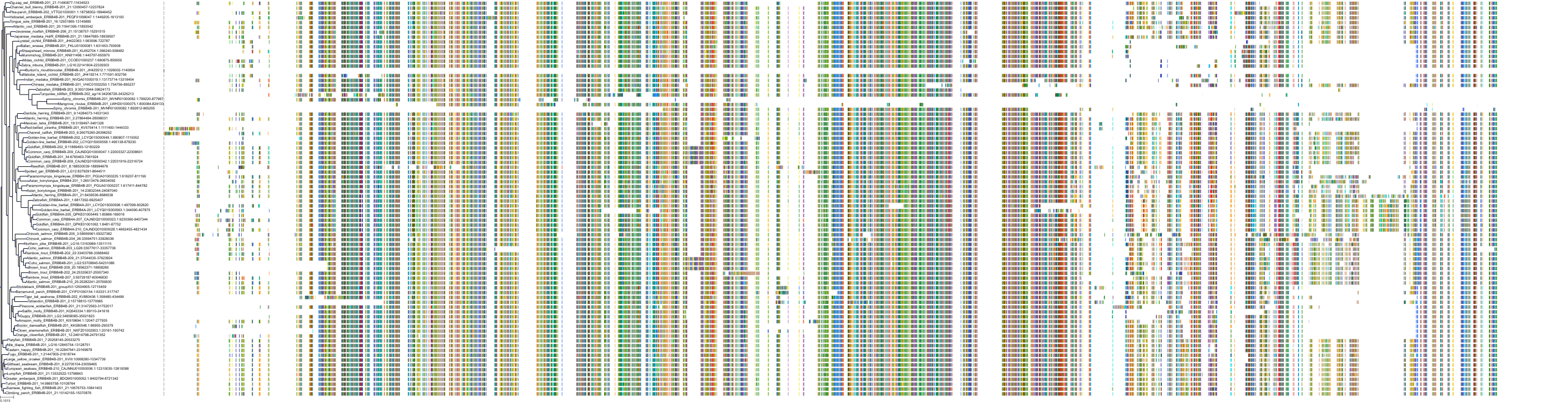
Target Conservation
|
Protein: Epidermal growth factor receptor erbB1 Description: Epidermal growth factor receptor Organism : Homo sapiens P00533 ENSG00000146648 |
||||
|
Protein: Receptor protein-tyrosine kinase erbB-2 Description: Receptor tyrosine-protein kinase erbB-2 Organism : Homo sapiens P04626 ENSG00000141736 |
||||
|
Protein: Receptor protein-tyrosine kinase erbB-4 Description: Receptor tyrosine-protein kinase erbB-4 Organism : Homo sapiens Q15303 ENSG00000178568 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 76003 |
| ChEMBL | CHEMBL2105712 |
| FDA SRS | V1T5K7RZ0B |
| Guide to Pharmacology | 5667 |
| KEGG | D09724 |
| PDB | 0WM |
| PubChem | 15606394 |
| SureChEMBL | SCHEMBL17764192 |
| ZINC | ZINC03976838 |